Dr Tim J. Wooster, Professor Mary A. Augustin, Food Science Australia (CSIRO)of Chemistry, Monash University, Victoria 3800, Australia. (This application note is an excerpt taken from T.J. Wooster and M.A. Augustin(2006) J. Colloid Interface Sci. 303, 564)
This application note discusses the effectiveness of protein-carbohydrate conjugates as steric stabilizers by studying the susceptibility of emulsions to flocculation in high electrostatic screening environments.
Caseins and whey proteins have been widely used as emulsifiers in various applications. The caseins form a thick fluid interfacial layer which extends 13 to 15nm into the bulk aqueous phase [1]. Whey proteins emulsions are stabilized by electrostatic charge [2].
Several studies have reported improved emulsion capacity and stability when proteins have been chemically linked to carbohydrates [3-5]. Whey protein emulsion stability can be enhanced by attaching a steric layer through reaction with a polysaccharide via a carbodiimide linkage or the Maillard reaction [1]. β-lactoglobulin can be chemically linked to dextran and this is a good model for studying how bulky the attached carbohydrate has to be in order to impart high steric stability to emulsions.
In a related application note called Determining Steric Layer Thickness and Conformation Using Dynamic Light Scattering (MRK1001-01), the preparation of a number of β-lactoglobulin-dextran conjugates was described. The thickness of adsorbed layers of these conjugates onto latex particles as a function of the change with dextran molecular weight was discussed.
The results showed that the attachment of dextran to β-lactoglobulin increased steric layer thickness. There was a high correlation between the increase in thickness and hydrodynamic diameter suggesting that there was minimal change to dextran structure upon attachment [6].
Extensive details of the preparation, evaluation of the extent of conjugation and purification of β-lactoglobulin-dextran conjugates can be found in the literature [6].
Emulsions were prepared by homogenizing 20 wt% canola oil and 80 wt% aqueous protein/conjugate emulsifier solution at room temperature. In all cases, the aqueous emulsifier solution contained 1 wt% β-lactoglobulin. In the case of conjugate emulsifier, this protein was conjugated to dextran. A coarse emulsion was prepared first by blending the components with an Ultra-turrax mixer at 13,500 rpm for 2 minutes. A fine emulsion was then prepared by passing this through a valve homogenizer (5 passes at 80 bar). Conjugates used for emulsification were not purified and contained unreacted dextran and unreacted β-lactoglobulin The effect of calcium concentration (0 to 20mM) on emulsion stability was assessed by diluting the previous emulsions to 10 wt% oil with CaCl2 solutions (at pH 7.0)and measuring the emulsion and flocculent size after exposure to CaCl2 for 24 hours. 0.02 wt% sodium azide was added to all emulsions after formation to prevent microbial contamination.
Emulsion particle size was assessed by laser light scattering using a Malvern Mastersizer 2000. Samples were diluted with distilled water to approximately 0.002 wt% to avoid multiple scattering effects. Information about emulsion particle size was obtained via a best fit between light scattering theory and the measured light scattering pattern. Emulsion particle sizes are quoted as the surface area mean diameter d3,2 (d3,2 = Σnidi3/Σnidi2) or the volume-mean diameter d4,3 (d4,3 = Σnidi4/Σnidi3). Particle size results are an average of three measurements of two freshly prepared emulsions. Standard deviations reported in these figures represent the standard deviation of three measurements on the one preparation.
Emulsion zeta potential measurements were conducted using a Malvern Zetasizer Nano ZS instrument. Prior to analysis, emulsions were diluted to 0.05 wt% using buffer solutions at the same pH and salt concentration as the parent emulsion. Emulsion zeta potentials were obtained by measuring the direction and velocity of droplet movement when subjected to an applied electric field. Sample zeta potentials were assessed using the folded capillary cell and are an average of three measurements on two separate emulsions. Standard deviations on the graph represent triplicate measurements on the same sample.
The preparation of various β -lactoglobulin-dextran conjugates has been discussed in an application note previously mentioned above. These various conjugates were used to enhance emulsion stability via steric stabilization. Table 1 summarizes the measured and calculated hydrodynamic dimensions of β-lactoglobulin and dextran in solution and conjugates as adsorbed layers used in this study.
| Material Properties | β-Lactoglobulin-Dextran Conjugate Properties | |||
|---|---|---|---|---|
| Molecular Weight (kDa) | Hydrodynamic Diameter (nm)a | Adsorbed Layer Thickness (nm)b | Dextran Steric Layer Thickness (nm)c | |
| β-lactoglobulin | 18.3 | 5.6 (dimer) | 2.9 | 0 |
| D20 | 18.2 | 5.0 | 8.2 | 5.3 |
| D35 | 36.0 | 8.0 | 12 | 9.1 |
| D65 | 60.3 | 10 | 15.7 | 12.9 |
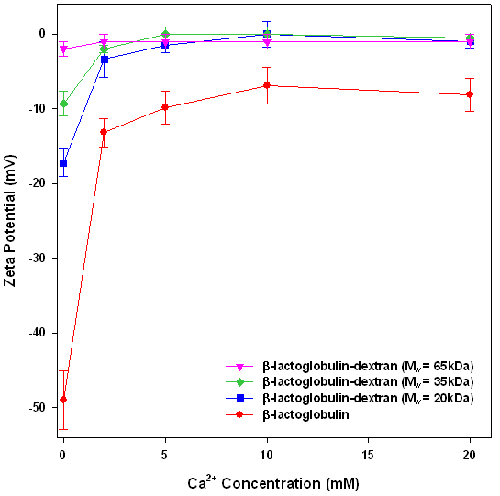
To test the stabilization conferred by the dextran layers, the stability of emulsions, formed from crude β-lactoglobulin-dextran conjugates, against calcium induced flocculation was assessed. The effect of calcium on the zeta potential and particle size of β-lactoglobulin and β-lactoglobulin-dextran conjugate emulsions are presented in figures 1 and 2 respectively.
In the absence of calcium chloride, the emulsions had a negative charge, indicating that the counter ion should be Ca2+.
Figure 1 shows that the zeta potential of each emulsion decreases with increasing concentration of calcium, confirming that calcium addition leads to loss of emulsion charge via a combination of electrostatic screening and calcium ion binding to the emulsion surface. Interestingly, in the absence of added calcium chloride, the charge of the emulsion droplets decreases with the increase in the molecular weight of the attached dextran. This may be attributed to the addition of the dextran layer which is physically screening the protein charge. Zeta potential is measured at the slipping plane of the emulsion. In the emulsions stabilized by conjugates, the slipping plane is the edge of the dextran layer. However, the charge is on the protein which is several nanometers away from the slipping plane. As the dextran layer gets thicker with increasing molecular weight, the distance between the protein charge and the slipping plane increases, therefore reducing the observed emulsion charge.
|
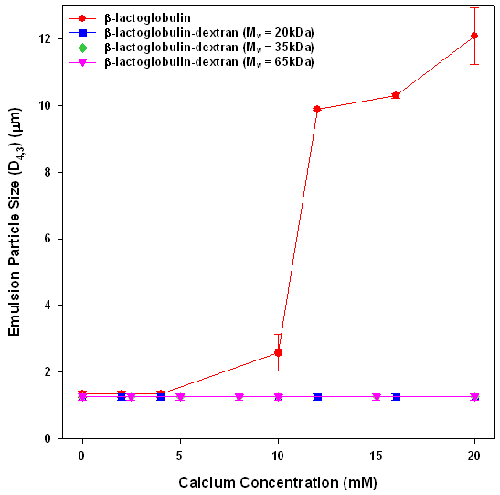
The effect of calcium addition on emulsion stability was assessed by measuring the particle size of emulsions stabilized by β-lactoglobulin and β-lactoglobulin-dextran conjugates after exposure to calcium for 24 hours (figure 2). Whilst all emulsions have similar initial sizes (D4,3 = 1.25μm ± 0.1μm) , they exhibited different aggregation behavior on exposure to calcium chloride for 24 hours. The β-lactoglobulin emulsions were unstable to calcium concentrations above 8mM, which corresponds well with what other researchers have found [7]. The emulsions formed from β-lactoglobulin-dextran conjugates showed no size increases at any calcium concentration. The observed emulsion stability must arise from the introduction of steric stabilization by the dextran layers.
|
These results show that increasing the interfacial adsorbed layer from 2.9nm for the β-lactoglobulin stabilized emulsion to 8.2nm by the conjugation of a low molecular weight dextran to the protein, stabilized emulsions against flocculation. This confirms the DLVO prediction [8] that adsorption of a 5nm thick steric layer (when sufficiently dense) imparts excellent colloidal stability to β-lactoglobulin emulsions.
This work found that the attachment of dextran to β-lactoglobulin increased steric layer thickness resulting in increased emulsion stability against calcium induced flocculation. All emulsions formed using β-lactoglobulin-dextran conjugates were stable against calcium flocculation, suggesting that these dextran steric layers were sufficient to provide high emulsion stability. These results indicate that an increase of 5nm in the steric layer by the attachment of dextran was sufficient to obtain effective steric stabilization against Ca2+ induced flocculation. The results obtained found that the steric layer size was controlled by the dextran molecular weight, suggesting that the results of layer thickness and emulsion stability will be universal across all globular proteins.
[1] E. Dickinson (1997) J. Dairy Sci. 80, 2607.
[2] S. Tcholakova, N.D. Denkov, D. Sidzhakova, I.B. Ivanov and B. Campbell (2005) Langmuir 21, 4842.
[3] L. Jimenez-Castano, R. Lopez-Fandino, A. Olano and M. Villamiel (2005) Food Chem. 93, 689.
[4] A. Kato (2002) Food Sci. Technol. Res. 8, 193.
[5] C.A. Dunlap, G.L. Côté (2005) J. Agric. Food Chem. 53, 419.
[6] T.J. Wooster and M.A. Augustin (2006) J. Colloid Interface Sci. 303, 564.
[7] E. Keowmaneechai and D.J. McClements (2002) J. Agric. Food Chem. 50, 7145.
[8] S. Damodaran (2005) J. Food Sci. 70, R54-R66.