Formulation development is a critical activity for many industries, from pharmaceuticals, to paints and coatings. Indeed, it is estimated that in the UK alone, sales of formulated products amount to some £180 billion per year1. This means that getting formulation ‘right’ is an important goal, with considerable value attached to it. Stability is a crucial attribute for many products with a direct impact on performance, shelf-life, kerb appeal and ultimately, worth. A systematic approach to understanding and developing stability can therefore be extremely helpful in improving the likelihood of success in long-term stability trials and accelerating formulation.
The Zetasizer range from Malvern Panalytical is the world’s most widely used system for nanoparticle, colloid and protein size and zeta potential measurements. In this whitepaper, we examine how these capabilities can be exploited to develop stable dispersions.
Formulation development is a critical activity for many industries, from pharmaceuticals, to paints and coatings. Indeed, it is estimated that in the UK alone, sales of formulated products amount to some £180 billion per year1. This means that getting formulation ‘right’ is an important goal, with considerable value attached to it. Stability is a crucial attribute for many products with a direct impact on performance, shelf-life, kerb appeal and ultimately, worth. A systematic approach to understanding and developing stability can therefore be extremely helpful in improving the likelihood of success in long-term stability trials and accelerating formulation.
The Zetasizer range from Malvern Panalytical is the world’s most widely used system for nanoparticle, colloid and protein size and zeta potential measurements. In this whitepaper, we examine how these capabilities can be exploited to develop stable dispersions.
When it comes to suspensions and emulsions, stability refers to the tendency of the dispersed phase to remain unchanged and uniformly distributed through the continuous liquid phase over time. Primary mechanisms of instability include aggregation or coagulation, the joining together of one of more particles, and sedimentation or settling. These mechanisms may occur independently, or in combination, as in the case of aggregation followed by associated sedimentation of the resulting larger particles – a common problem.
The defining goal of formulation is to optimize properties to meet a specific application. If a resulting formulation is unstable, these optimized characteristics are easily eroded or lost. For example, in a suspension medicine, the size of the dispersed drug particles is associated with in vivo dissolution and bioavailability, while a homogeneous particle distribution ensures uniform dosing. If the drug particles aggregate and/or sediment, both clinical efficacy and safety may be compromised. On the other hand, in a paint or ink, dispersed particle size influences the way in which light interacts with the finished coating – observed hue or tint, transparency and/or level of gloss – and practical features such as weather resistance and ease of application. Here, aggregation impacts directly upon consistency, performance and value.
Stability testing is a key aspect of formulation. It defines the period of time over which a customer may expect the product to deliver consistent, optimized and/or safe performance, thereby setting a specification for shelf life. Understanding and controlling stability is the key to extending shelf life as far as possible to maximize the practicality, safety and commercial appeal of the product. The following sections detail five strategies that can be usefully adopted with the Zetasizer Nano to achieve that goal.
Measuring particle size at or near to neat sample concentrations detects the onset of aggregation under relevant conditions.
The size of particles in suspension is not necessarily the size of the particles that were used to make up the suspension. Measuring the size of particles in the formulation, undiluted, detects any tendency towards aggregation under ‘in-use’ conditions.
The Zetasizer Nano measures particle size across the range 0.3 nm to 10 µm using the technique of dynamic light scattering (DLS). Small particles in a dispersion/suspension are subject to Brownian motion. The speed of Brownian motion can be measured directly from the scattered light pattern produced by the moving particles. DLS systems determine the rate of diffusion of particles from measurements of fluctuations in the intensity of light scattering and convert the result to particle size/particle size distribution data via the Stokes Einstein equation. Because light scattering intensity correlates with particle size to the power six, DLS is highly sensitive to the presence of low levels of larger particles or aggregates.
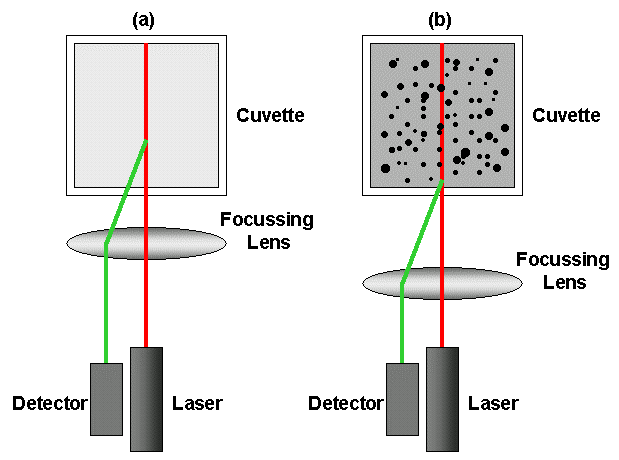
Figure 1: Schematic showing the key components of a DLS system – laser, detector, focusing lens and cuvette (which holds the sample) – in a NIBS configuration
Non-invasive backscattering (NIBS) technology – detecting scattered light at a large angle relative to the incident beam (173° in the case of the Zetasizer Nano) increases sensitivity and extends the concentration range over which accurate size data can be gathered by reducing multiple scattering. Multiple scattering is where the light interacts with more than one particle prior to detection. NIBS technology within the Zetasizer Nano also includes high quality movable optics that automatically focus the lens to maximize accuracy for every sample. The net result is accurate, relevant particle size data over a concentration range that runs from 0.1 ppm to 40% w/v, enabling measurement of the broadest range of formulations without dilution.
Droplet size measurements of a pharmaceutical emulsion were made across a range of sample concentrations, from 0.0001 to > 5 %w/v, to assess any effect of concentration on recorded values (see figure 2). The results show that measured droplet size data is largely independent of sample concentration confirming the ability of the instrument to deliver reliable data as sample concentration, and the potential for back scattering, increases.
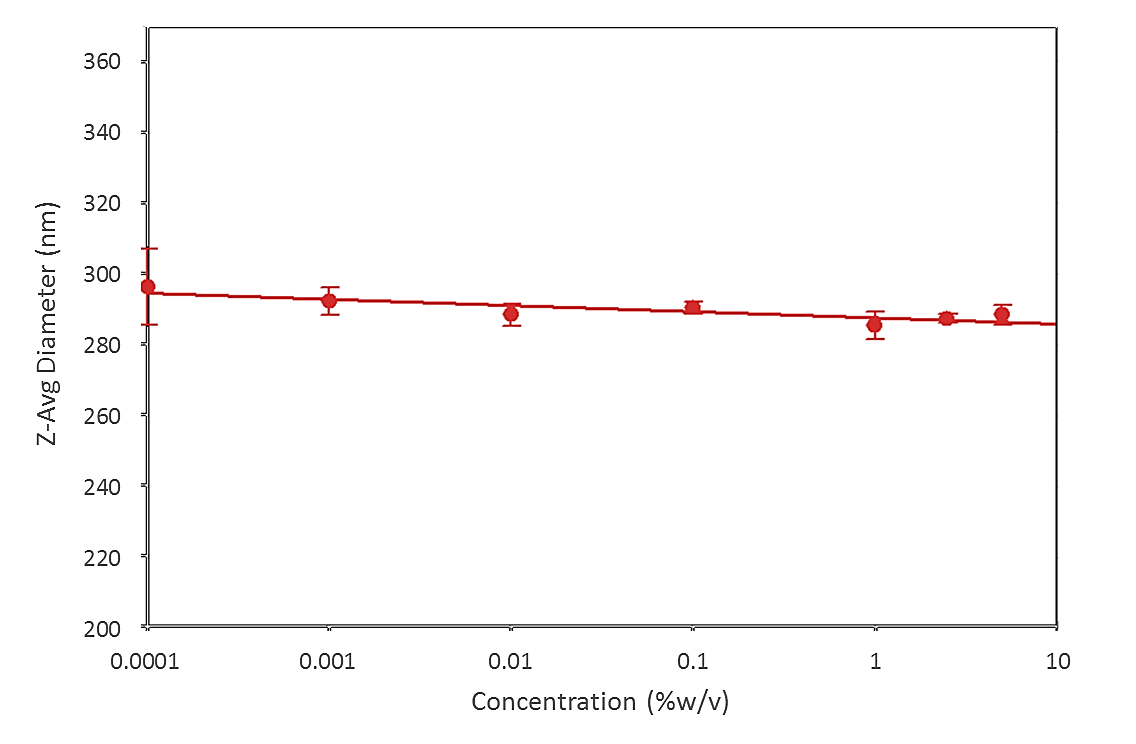
Figure 2: Data for a pharmaceutical emulsion show the ability of the Zetasizer Nano to reliably and repeatably measure particle size over a wide range of sample concentrations, using NIBS technology
Zeta potential is directly related to electrostatic stability, providing the information needed to optimize the effect of charge within a dispersion.
Zeta potential is a measure of the magnitude of electrostatic forces of repulsion or attraction between particles in a dispersion. If particles repel then the risk of aggregation and instability is reduced. A zeta potential of low magnitude, most especially relatively close to 0, indicates that particles are likely to attract and consequently aggregate. A zeta potential of high magnitude, ideally greater than +/- 30 mV, is associated with greater stability.
Zeta potential is measured using the technique of electrophoretic light scattering ( ELS) or laser Doppler micro-electrophoresis. When an electric field is applied to a dispersion/suspension, any charged particles present move in a direction and at a velocity associated with their zeta potential (see box). Measurements of velocity enable the calculation of electrophoretic mobility, and from this, zeta potential.
A configuration for both DLS and ELS measurements – the Zetasizer Nano is configured to measure both particle size and zeta potential, increasing its value for stability assessment.
M3-PALS technology – accurate measurement of the speed of particle movement is essential for the precise determination of zeta potential. The Zetasizer Nano uses a patented laser interferometric technique called M3-PALS (Mixed Mode Measurement - Phase Analysis Light Scattering) to deliver exceptional sensitivity2. M3-PALS allows samples with low electrophoretic mobility to be analyzed and their mobility distributions precisely resolved.
Silicones exhibit a high degree of thermal stability, and are consequently integrated in a wide range of household and medical products in the form of emulsions. Measurements of the zeta potential of three different silicone emulsion samples were compared with observed stability rankings to determine whether zeta potential could be used to predict the stability of a fourth sample.
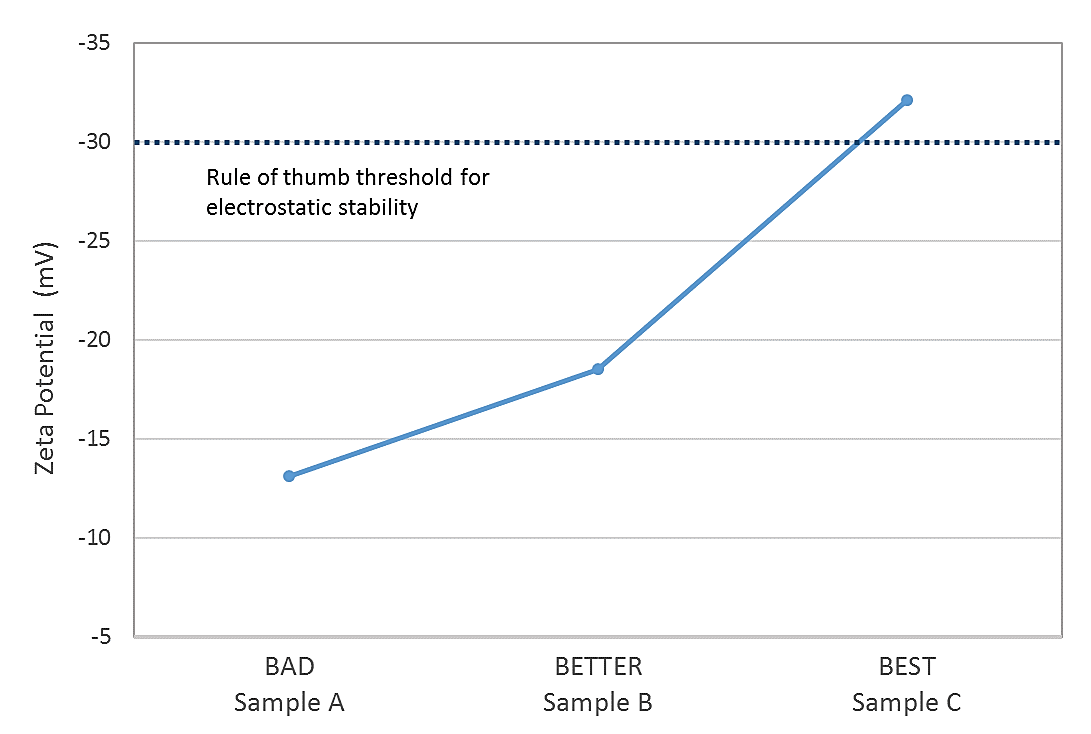
Figure 3: Data for silicone emulsion samples show a close correlation between observed stability and zeta potential values
The results show that there is a close correlation between measured zeta potential and observed stability with only one sample (#3) exhibiting a zeta potential indicative of robust electrostatic stability (see figure 3). From the results the stability of the fourth sample, which had a measured zeta potential of 13.2 mV, can be predicted to have poor stability, closely similar to that of sample A.
Measuring the DLS interaction parameter, kD, alongside zeta potential supports the robust prediction of long term suspension stability.
The DLS interaction parameter (kD) is affected by both thermodynamic and hydrodynamic interactions between particles in a suspension and is a measure of the propensity of a larger molecule to separate reversibly into smaller components – the opposite of aggregation. A more positive kD indicates strong repulsive interactions and, correspondingly, greater stability. Measurements of kD under relevant conditions therefore add to the insight into formulation stability provided by zeta potential.
kD is calculated from the concentration dependence of the diffusion coefficient of the sample, as determined from DLS data using the following expression,
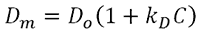
where Dm is the mutual (measured) diffusion coefficient, D0 is the self-diffusion coefficient (the diffusion coefficient at zero concentration), and C is the sample concentration. It can therefore be easily determined via a series of DLS measurements.
Advanced software – the Zetasizer Nano has software that calculates KD from test measurements, easing access to the data required.
Human Serum Albumin (HSA) dispersions are deployed in a range of medical applications, to, for example, restore blood volume, and are used routinely as a biological reference standard. The impact of pH on the stability of HSA dispersions was determined by generating values of kD at a pH of 7 and 4, via dynamic Debye plots of diffusion coefficient as a function of concentration (see figure 4). The resulting data indicate that stability is improved by increasing the acidity of the dispersion.
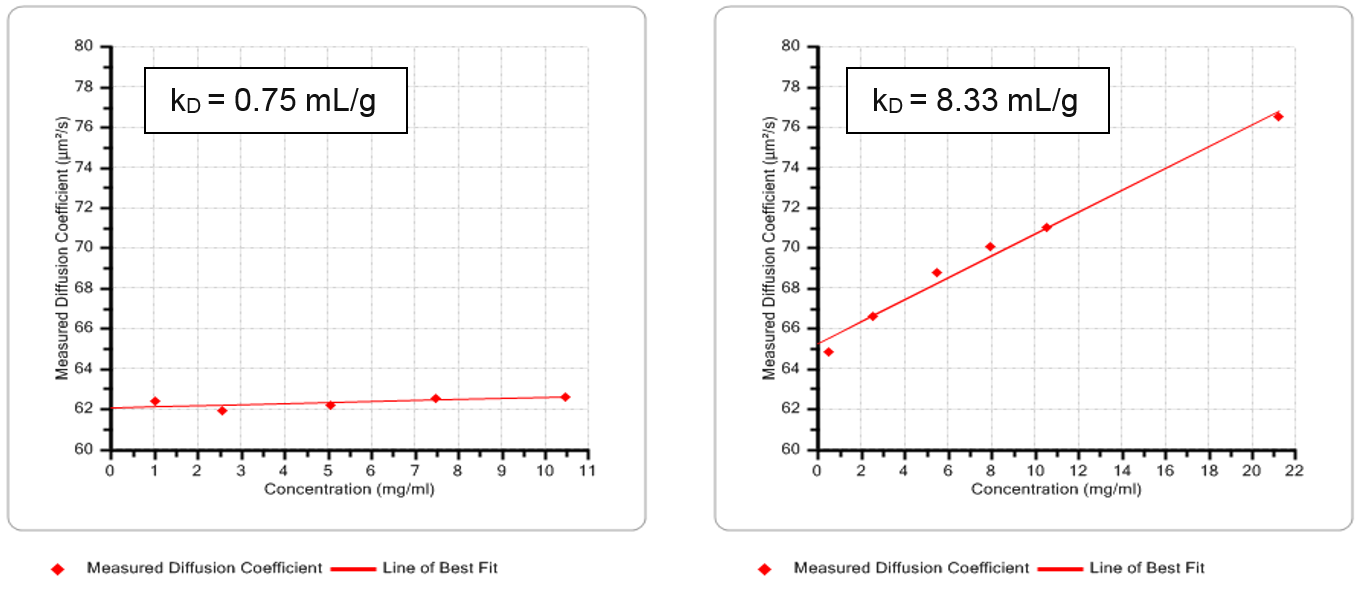
Figure 4: Dynamic Debye plots for HSA at pH 7 (left) and pH 4 (right) with lines of best fit for determining kD. The results show that the dispersion is more stable under more acidic conditions
Efficiently scoping the influence on stability of pH, ionic strength and composition accelerates progress to a formulation that meets stability targets.
Knowledgably manipulating the composition of a formulation to meet stability targets requires an understanding of how different components may impact stability. Measuring zeta potential and/or particle size as a function of the parameter of interest is an effective alternative to a trial and error approach.
The impact of parameters, such as pH and ionic strength, requires repeat measurements of samples that vary in terms of the parameter of interest. Automation can help to substantially reduce the manual input required for such testing, and at the same time enhance instrumental productivity.
An autotitrator - such as the MPT-2 integrated titrator accessory for the Zetasizer Nano, performs automatic pH, conductivity and additive titrations, thereby accelerating stability investigations. The MPT-2 automatically changes the composition of the sample before transferring it to the optics unit for the measurement of particle size and zeta potential. It also enables automated measurement of the isoelectric point (IEP) – the point of zero zeta potential.
Intralipid is a triglyceride oil-in-water emulsion used for parenteral nutrition either alone or mixed with other components, such as vitamins and minerals, to form a total parenteral nutrition (TPN) solution. Figure 5 shows a plot of the zeta potential of Intralipid emulsions as a function of calcium chloride, which was used to simulate the effect of addition of a mineral. The results show that the Intralipid exhibits high stability in the absence of calcium chloride– a zeta potential value of around -50 mV. However, this stability is quickly eroded through the addition of even small quantities of calcium chloride, with an IEP observed at a concentration of around 4 mM. Further additions of calcium chloride cause a charge reversal in zeta potential, suggesting that the Ca2+ ions bind to the surface of the emulsion droplets.
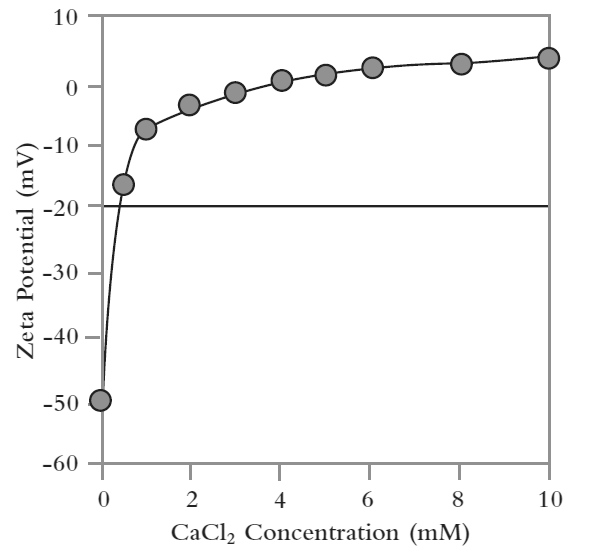
Figure 5: A plot of zeta potential as a function of calcium chloride concentration shows how the addition of the salt compromises stability
Temperature can directly impact formulation stability, making it essential to scope any effect.
Elevated temperatures can change formulation stability by, for example, reducing the viscosity of the continuous phase such that sedimentation is more likely to occur, or accelerating particle movement, increasing the likelihood of aggregation. Investigating the impact of temperature is therefore important for formulations destined for use or storage at elevated temperatures. More broadly, however, testing at elevated temperature can be a useful strategy for differentiating the stability of closely similar formulations.
As with assessments of the impact of composition, exploration of the effect of temperature simply requires repeated measurements under closely controlled conditions with automation useful in alleviating the analytical burden.
Built in, high performance temperature control – an efficient alternative to an external water bath and circulation system and will give better results. Good temperature control is an essential requirement for all DLS measurements, since the Brownian motion of a particle and the viscosity of the dispersant are both directly dependent on temperature. The Zetasizer Nano offers integral Peltier temperature control over the range 0 to 90°C to +/-0.1°C, as well as fast system warm-up and the ability to rapidly change temperature – both features that can be useful for elevated temperature studies.
The zeta potentials of two paint samples (#305 and #803) were measured to quantify stability. The samples were synthesized using different chemical polymerization methods and, in use, #803 was found to be stable, while #305 was not, with a deposit forming in the paint after a few months. The zeta potential values were for the two samples were 55.0 and 51.1 mV respectively and neither these results, nor size data measured at ambient temperature, provided any indication as to the why the paints might perform differently. Repeat measurements of size were carried out at 80°C to see if higher temperature testing would differentiate them (see figure 6).
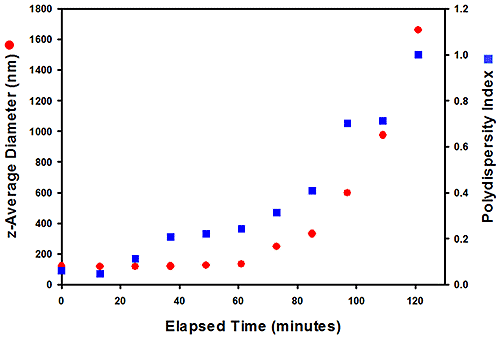
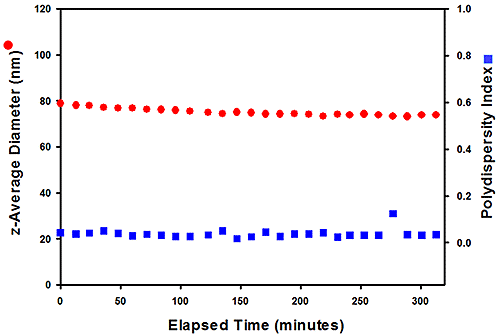
Figure 6: Size data for the #305 sample (top), measured at 80°C, show that it aggregates over time, while #803 (right) has greater stability
The results measured at 80°C show that, after around 40 minutes, sample #305 begins to aggregate, while the size of particles in sample #803 remains constant over four hours, clearly demonstrating its superior stability. Here then, testing correctly identifies an issue a difference in stability over a relatively short timescale, relative to the months of storage required to observe an effect in use.
Introducing zeta potential: a reliable indicator of dispersion stabilityMeasurements of zeta potential are commonly used to assess the stability of a wide variety of colloidal systems. Zeta potential is a measure of the magnitude of electrostatic or charge repulsion at the boundary layer surrounding a particle and is one of the fundamental parameters known to affect a system’s stability. Two layers of ions surround particles within a solution, the tightly bound inner Stern layer and the outer diffuse layer, where ions are less firmly associated. Within the diffuse layer there is a notional boundary called the slipping plane beyond which the particle exerts little influence. Charge at the slipping plane is referred to as the zeta potential.
Zeta potential therefore quantifies the balance of repulsive and attractive forces that the particles experience as they approach one another. At a zeta potential near to zero, these forces are low and the system is unstable, highly prone to aggregation. A pronounced negative or positive zeta potential (+/-30 mV), on the other hand, is indicative of an electrostatically stable system that will resist particle aggregation. |
1. https://connect.innovateuk.org/web/formulation1
2. Simplifying the measurement of zeta potential using M3-PALS - Available for viewing at the Malvern Panalytical website: https://www.malvernpanalytical.com/en/learn/knowledge-center/application-notes/AN101104ZetaPotentialM3-PALS.html
For formulators, the Zetasizer Nano offers multiple measurement protocols that support an efficient knowledge-led approach – in place of trial and error – and swift progress towards an optimized formulation that meets performance targets. To learn more about the value of Zetasizer Nano for your application please read on in the following areas:
DLS: An introduction in 30 minutes – Available for viewing at the Malvern Panalytical website: https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/TN101104ZetaPotentialIntroduction.html
Zeta potential: An introduction in 30 minutes – Available for viewing at the Malvern Panalytical website: https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/TN101104DynamicLightScatteringIntroduction