Particle size is an important parameter in any pharmaceutical product. This is primarily because the particle size and distribution are directly related to the dissolution rate of the active pharmaceutical ingredient (API). The particle size of the API and excipients present in suspension-based products may also affect physical characteristics such as the product's viscosity, and therefore mouth feel, and its stability over time.
This application note describes the characterization of two indigestion treatments, both of which are suspension-based formulations. One product claims to be fast acting, the other long lasting whilst both have the same composition (Table 1). The study aims to link the particle size of the ingredients to the product's solubility (and hence bioavailability) and the rheology of suspensions.
Particle size is an important parameter in any pharmaceutical product. This is primarily because the particle size and distribution are directly related to the dissolution rate of the active pharmaceutical ingredient (API). The particle size of the API and excipients present in suspension-based products may also affect physical characteristics such as the product's viscosity, and therefore mouth feel, and its stability over time.
This application note describes the characterization of two indigestion treatments, both of which are suspension-based formulations. One product claims to be fast acting, the other long lasting whilst both have the same composition (Table 1). The study aims to link the particle size of the ingredients to the product's solubility (and hence bioavailability) and the rheology of suspensions.
Table 1: Ingestion liquid compositions for fast-action and long-acting products
| Fast- acting (10ml) | Long- lasting (10ml) | |
| Sodium alginate (mg) | 500 | 500 |
| Sodium bicarbonate (mg) | 267 | 267 |
| Calcium carbonate (mg) | 160 | 160 |
| Carbonates (mg) | 427 | 427 |
| Sodium (mmol) | 6.2 | 6.2 |
The particle size of the API present within a drug product can have a direct influence on the functionality of a product. Indeed, the FDA recommend that particle size analysis is carried out for all solid, suspension or emulsion based products if the particle size is know to be critical to [1]:
If any of the above is important in determining the activity of the product, then sponsors are required to set particle-size based specifications.
The dissolution rate of an API is critical as is directly related to the time of release into the bloodstream. The relationship between the drug particle surface area and the dissolution rate is described by the Noyes-Whtiney equation:
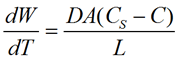
|
Where
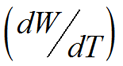
|
is the dissolution rate, A is the surface area of the solid, C is the concentration of the solid in the bulk dissolution medium, CS is the concentration of the solid in the diffusion layer surrounding the solid, D is the diffusion coefficient and L is the diffusion layer thickness.
The important information to take from this equation is the direct relationship between the dissolution rate and the surface area of the solid, which itself is dependent on the particle size. As the particle size reduces, so the surface area will increase. This would be expected to increase the dissolution rate of the API and therefore how rapidly the drug becomes bioavailable.
The stability and dose content uniformity of a drug product may be related to not only the particle size but to the overall particle size distribution as well. If the suspension is polydisperse or contains very large particles (which themselves contain a significant mass of drug) then it will be more difficult to deliver a uniform dose. The product may also be more susceptible to instability via either sedimentation or creaming. It is therefore important to be able to control the particle size and distribution width in order to deliver a repeatable dose.
|
Along with the bioavailability, uniformity and stability of a product, the customer perception of the product is also very important. In particular, the mouth-feel of suspension based products can be significantly affected by the particle size distribution of the API and any excipients.
First, particles of too large a size (greater than 20-30 microns) can be detected by the tongue and this will make the liquid feel gritty and unpleasant to drink. Second, the rheology of the suspension will be affected if the size distribution is broad or the fine particle fraction is high. This, in turn, can affect the ease with which the product can be swallowed.
The particle size of suspension-based products, such as indigestion liquids, will therefore need to be accurately measured and controlled in order to balance the requirements of bioavailability, stability, dose uniformity, storage and mouth-feel.
The experiments reported in this application note include the particle size and rheological analysis of two indigestion liquids; one fast-acting and one long-lasting. Both samples have the same ingredients in similar proportions. The ingredients are mainly calcium carbonate and sodium bicarbonate suspended in a sodium alginate solution, as shown in table 1.
Particle size analysis was carried out by laser diffraction using the Mastersizer. Laser diffraction is an extremely well established technique for particle sizing, and as such is covered by an international standard [2]. General advice on the use of the technique is also provided in the US and European pharmacopeia [3]. Some of the advantages of laser diffraction include its wide dynamic range and flexibility to measure dry powders, suspensions and emulsions. Laser diffraction measurements also capture a large number of particles, providing statistical significance for each measurement even when measuring very polydisperse distributions. The measurements are also fast, allowing many samples to be analyzed in a day.
Laser diffraction instruments work on the principle that large particles scatter light at small angles and small particles scatter light at large angles. The Mastersizer measures the light energy scattered by a sample as a function of angle. This data is then compared against an appropriate scattering model in order calculate the size distribution of the sample.
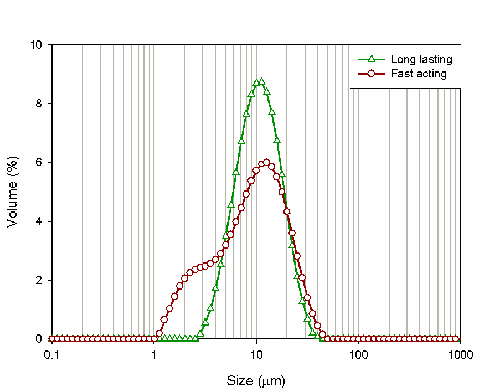
For this study, both of the indigestion relief liquids were dispersed in de-ionized water prior to measurement using the Mastersizer system. Table 2 gives the particle size results reported by the system. Looking at the Dv50 and the Dv90, there is not a huge difference between the samples; the only noticeable difference is that the Dv10 of the fast acting liquid is 3 microns smaller than the Dv10 of the long lasting liquid.
Table 2: Particle size of both samples
Sample | Dv(10) μm | Dv(50) μm | Dv(90) μm |
| Long-lasting | 5.103 | 9.889 | 18.649 |
| Fast-acting | 2.310 | 8.877 | 20.811 |
The difference between the samples becomes much more noticeable when the particle size distributions are compared. Figure 1 shows the distributions reported for both of the products: the red curve relates to the long lasting product whereas the green curve relates to the fast acting product.
The fast acting product shows a shoulder of fine particles. These particles will be rapidly solubalised following administration and may therefore be responsible for the claims made regarding the fast onset of relief associated with the product. Conversely, the larger particle size of the long-acting product will lead to slower dissolution of the active. This may explain why this product will deliver indigestion relief for a longer period of time.
As well affecting the rate of absorption, the particle size distribution of each of the indigestion products will affect their appearance. This is best assessed by analyzing the rheological properties of the suspensions, as this allows prediction of the stability of the product during storage as well as providing a measure of how patients may perceive the product. A high viscosity will give the product stability during storage as particle sedimentation will be inhibited. However, a high viscosity is not desirable when it comes to the product usage as viscous liquids are unpleasant to drink.
Measurements of the rheological properties were carried out using the Bohlin Gemini HR Nano rheometer. The flow behavior of the material was determined under a range of conditions by measuring the shear viscosity as a function of shear rate over a shear rate range from 0.001s-1 to 100s-1.
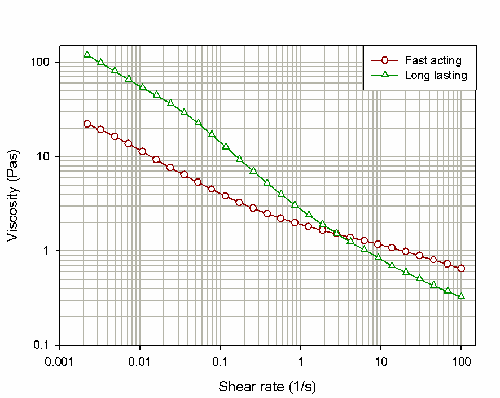
Figure 2 shows the flow curves obtained for both products. These results suggest that at low shear rates, the long lasting product has a higher viscosity than the fast acting product. Differences in viscosity at low shear rates are generally caused by weak interactions between any particulate or polymeric species
|
present within the formulation. These interactions are overcome within the long-acting product as the shear rate is increased, such that the high-shear viscosity for this product is less than that reported for the fast acting product.
The rheological properties of the suspensions can be related to the particle size distributions shown in figure 1. The presence of the shoulder of fine particles within the fast acting product leads to its maximum packing fraction being higher compared to the long-acting product. This is due to the fact that the fine particles are able to pack into the gaps around the larger particles within the suspension. The low shear viscosity is related to this packing fraction via the Kreiger-Doherty equation, such that:
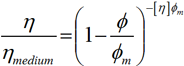
|
where η is the viscosity of the suspension as a whole, ηmedium is the viscosity of the base liquid, φ is the volume fraction of solids in the suspension, φm is the maximum packing faction of solids in the suspension and [η] is the intrinsic viscosity of the medium (2.5 for spheres). This equation predicts that, for a fixed volume fraction, the suspension viscosity will decrease as the maximum packaging fraction increases. As the volume fraction of the constituents is the same for both indigestion products (table 1), this equation predicts that the higher maximum packaging fraction associated with the fast acting product will yield a lower viscosity. This can also be thought of as being due to the smaller particles acting like a lubricant and allowing the larger particles to move past each other more freely.
At high shear rates the fast acting product has a higher viscosity compared to the long lasting product. The differences in viscosity at higher shear rates must be due to a different type of interaction, as the weak interactions present at low shear rates are broken down by the application of shear. The differences observed here are instead due to the strong, attractive Van der Waals forces which exist between the particles in suspension. Van der Waals forces increase as the particle surface area increases and will therefore become more dominant as the particle size decreases. These interactions will therefore be more dominant within the fast acting product, yielding a higher viscosity at high shear rates compared to the long-acting product.
According to the rheological properties described above, the long acting product has more desirable properties for both the manufacturer and the user. When the product is being stored (i.e. at low shear rates) the product has a higher viscosity - this will make the product more stable. However, this viscosity is reduced at higher shear rates (e.g. during shaking of the product or during administration), making the liquid thinner and therefore more pleasant to drink.
Particle size distributions and rheological flow curves have been obtained for two indigestion relief liquids; one claiming to be fast acting the other long lasting. In this application note we have used the particle size distributions to explain the rheological properties and therefore the product's physical characteristics.
The particle size distribution required for a pharmaceutical suspension, such as an indigestion liquid, is a delicate balance between achieving the required dissolution rate (bioavailability), stability and product characteristics. As such it is important to characterize and control the particle size distribution. Increasing the proportion of fine particles will cause dissolution to occur more rapidly but may also lead to the development of undesirable rheological properties.
[1] FDA Draft CMC Guidance for Industry; Analytical Procedures and Methods Validation; Section XI; Part F (Aug 2000).
[2] ISO 13320-1 Particle Size Analysis- Laser Diffraction Methods Part 1:General Principles (1999).
[3] See USP<429> and EP 2.9.31.