Lipid emulsions are used in a wide variety of pharmaceutical applications. These include intravenous delivery to patients with difficulty in ingesting food orally, the transport of lipophilic drugs, the controlled release of drugs and improved targeting of drugs [1].
Triglyceride oil-in-water emulsions are used for parenteral nutrition. They can be used alone or mixed with other essential components, such as amino acids, vitamins and minerals, in total parenteral nutrition (TPN) mixtures. These components can interact with the emulsion droplets causing flocculation and/or coalescence [2]. As a result a TPN mixture is prepared shortly before administration.
The zeta potential of a dispersion provides information of the long term stability of the sample. Studies of the influence of additives on the zeta potential of a sample can provide useful information for improvement of product formulation [3-5].
The zeta potential of a sample can be measured using electrophoretic light scattering on a Zetasizer Nano [6, 7]. In this technique, a laser beam is passed into a cell containing the sample and the scattered light produced by the particles is detected at a forward angle of 13°. This optical configuration imposes restrictions on the maximum turbidity of the sample which can be measured. If the concentration of the sample is too high, the laser beam will become attenuated by the particles reducing the scattered light that is being detected. Further discussion of the concentration limits for zeta potential measurements can be found in the technical note "Concentration Limits for Zeta Potential Measurements in the Zetasizer Nano" from the Malvern website [8].
The Zetasizer Nano is provided with default folded capillary cells (DTS1060C) for zeta potential measurement. The high concentration zeta potential cell ZEN1010 is an optional accessory which allows measurement of concentrated, more turbid samples due to a decreased path length.
Even though the ability to measure the zeta potential of samples at, or close to, their neat concentration is attractive to many users, there may be an influence of sample concentration on the results obtained. This concentration dependence needs to be studied and understood.
This application note discusses zeta potential measurements of Intralipid, a pharmaceutical emulsion, over a wide concentration range and discusses the results obtained with respect to sample concentration.
Experimental
The Intralipid used in this study was kindly provided by Fresenius Kabi at a neat concentration of 20% w/v. Various concentrations of the emulsion were prepared in 2.25% w/v glycerol with the pH adjusted to 7.9 with NaOH. This was to ensure that any change in the measured zeta potential was not due to changes in the dispersant conditions upon sample dilution.
All samples were measured on a Zetasizer Nano ZS at 25oC using the high concentration zeta potential cell ZEN1010. A field strength of 25V/cm was applied. Five repeat measurements were made on each sample to check the repeatability of the results obtained.
The viscosity of each sample concentration was determined on an A & D SV-10 vibro-viscometer at 25°C [9].
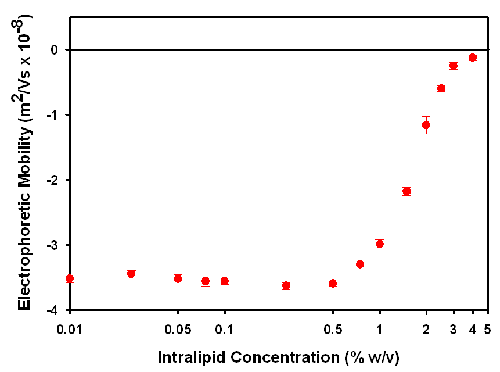
The technique of laser Doppler electrophoresis measures the electrophoretic mobility of the particles. Figure 1 is a plot of the electrophoretic mobility as a function of Intralipid concentration.
|
At concentrations below 0.5% w/v, the mobility is consistent at around -3.5 x 10-8 m2/Vs. As the concentration increases above 0.5% w/v, there is a gradual decrease in the mobility up to the maximum measureable concentration of 4%. At concentrations above this, the sample is so turbid that the laser beam becomes too attenuated by the presence of particles. This results in the intensity of scattered light being too low to be measured.
The decrease in the electrophoretic mobility can be due to a variety of reasons. It may result from a change in sample conditions with dilution. This is unlikely as the diluent used in this study should ensure that the sample conditions are maintained upon dilution. The reduction in electrophoretic mobility may also be due to an increase in viscosity as sample concentration increases. However, the variation in viscosity across the Intralipid concentration studied in this application note is less than 5% and does not account for the significant decreases seen in the results. The decrease in electrophoretic mobility is almost certainly due to the increase in sample turbidity. As the sample concentration increases, more light will be detected from particles near the wall than in the centre of the cell. These particles will have reduced mobility due to the electric field being lower than assumed. This results in a reduction in the mean electrophoretic mobility value.
The electrophoretic mobility (UE) is converted in zeta potential (z) using Henry's equation [3-5]:
UE = 2 ε ζ F(κa)/3η (1)
Where e is the dielectric constant of the dispersant, F(κa) is the Henry function and η is the viscosity.
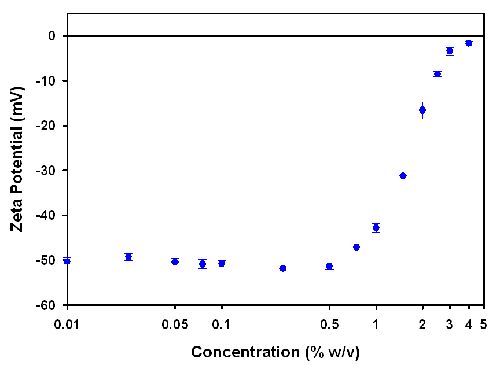
As can be seen from equation (1), the conversion from electrophoretic mobility to zeta potential requires the viscosity of the sample. The viscosities of the various concentrations were measured on an SV-10 vibro-viscometer and figure 2 shows the converted zeta potential values as a function of sample concentration.
|
The zeta potential values are consistent up to an Intralipid concentration of 0.5% w/v. Above this concentration, the zeta potential values decrease up to the maximum measureable concentration of 4%.
A previous study on the zeta potential of various Intralipid concentrations showed that the maximum measureable concentration in the folded capillary cell was 1% w/v [8]. The high concentration zeta potential cell used in this study allows Intralipid to be successfully measured up to concentrations of 4%. This four fold increase in the maximum measureable concentration is due to the reduction in the path length of the high concentration cell.
In both the folded capillary cell and the high concentration cell, the zeta potential values obtained at the maximum measureable concentration cannot be used as absolute values. However, for certain applications they can be used in a relative sense.
The high concentration cell accessory for the Zetasizer Nano range of instruments allows for zeta potential measurements to be made on turbid samples at high concentration. However, the influence of sample concentration on the zeta potential values obtained needs to be understood.
For any sample, measurements should be performed at a range of concentrations to identify a region where the zeta potential results obtained are independent of concentration. For such experiments, the dilution protocol is critical in ensuring that sample conditions are kept constant.
For samples which show a concentration dependence, the results obtained can still be used in a relative sense.
[1] Pharmaceutical Emulsions and Suspensions, Ed. F Nielloud and G. Marti-Mestres (2000) CRC Press.
[2] C. Washington, S. S. Davis and H. Kerbl (1993) J. Clin. Pharm. and Therapeutics 18, 351-355.
[3] Zeta Potential - An Introduction in 30 Minutes, Malvern Instruments Technical Note MRK 654-01.
[4] Hunter, R.J. (1988) Zeta Potential In Colloid Science: Principles And Applications, Academic Press, UK.
[5] Everett, D.H. (1994) Basic Principles of Colloid Science, The Royal Society of Chemistry, UK.
[6] Measuring Zeta Potential Using Laser Doppler Electrophoresis, Malvern Instruments Technical Note MRK 570-01.
[7] Measuring Zeta Potential Using Phase Analysis Light Scattering, Malvern Instruments Technical Note MRK 571-01.
[8] Concentration Limits for Zeta Potential Measurements in the Zetasizer Nano, Malvern Instruments Technical Note MRK 669-01.
[9] SV-10 Vibro-Viscometer, Malvern Instruments Technical Note MRK 650-01.