The formation of protein aggregates is a particular concern for parenteral administration biopharmaceuticals due to the potential for increased immunogenicity. As a consequence, there is an expectation from regulatory agencies for companies to monitor and, if required, reduce the levels of sub-visible particles present in therapeutic protein from manufacture through their complete shelf life. While immunogenicity can be induced by a variety of mechanisms, contamination by non-protein material is known to be a potential cause. The presence of silicone oil in parenterals has attracted considerable interest, due to its use in syringe-based administration systems and the difficulty in distinguishing oil droplets from protein aggregates. In addition to protein aggregates, non-biological contaminants may act as nucleation points for aggregate growth. Consequently, particle sizing alone is not sufficient.
Guidance from the FDA for the biopharmaceutical industry states “Naturally sourced products should be evaluated for other components, protein and non-protein” (1). Resonant Mass Measurement (RMM) technology in the Archimedes system measures the buoyant mass of particles passing through a cantilever, allowing detection of particles with a density different to that of the buffer solution. Consequently, the Archimedes provides the ability to distinguish between negatively buoyant particles, such as proteins, and positively buoyant particles, such as silicone oil. This application note provides an example of how Archimedes can be used to detect and quantify the formation of protein sub-visible particles and the introduction of silicone oil droplets, in response to shear stress.

|
Bovine Serum Albumin, BSA (Sigma Aldrich, Poole, UK), was prepared to a final concentration of 20 mg/mL in pure water, and filtered through a 0.2 µm syringe filter. Samples were analyzed pre and post shear stress. Shear stress was induced by repeatedly syringing the BSA solution through a 2 mL syringe and attached grade-23 needle. Analysis was performed using a micro sensor in the Archimedes Resonant Mass Measurement (RMM) system. Prior to running samples, the micro sensor was rinsed with pure water and calibrated using 1 µm latex beads and deuterium. Samples were run using a specified volume of 0.1 µL and a limit of detection of 0.02 Hz, with positive and negative buoyancy particles detected.
The impact of shear stress of the formation of protein aggregates and the introduction of silicone oil was determined using the Archimedes system. These data are shown below.
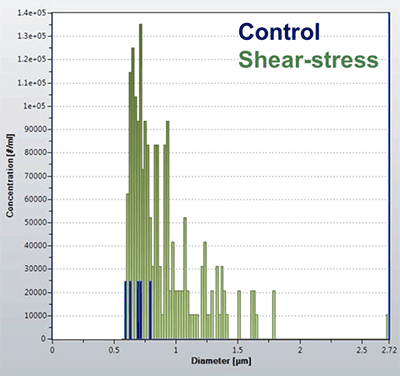
Sub-visible particles were quantified using RMM pre and post syringe-induced shear stress. These data are shown in Figure 2. Prior to syringe stress, the number of particles detected by the Archimedes system is very low, demonstrating the sample is reasonably pure, with very few large protein aggregates. This is to be expected, as the sample had been filtered. However, this does demonstrate the low noise baseline of the technique, enabling accurate analysis of pure samples. Following shear-stress the number of particles detected increases significantly, with particle sizes ranging from 500 nm up to 1700 nm. The particle size distribution provides important data regarding the response to the type of stress induced. Such data can be used to compare different stress conditions to provide an overall picture of the degradation profile for the biopharmaceutical of interest.
|
Understanding the impact of bioprocessing on protein characteristics is an important aspect of product knowledge and design of manufacturing parameters. To ensure compliance with regulatory requirements, the impact of materials used in the manufacturing process stream should be evaluated to ensure no impact on product quality (2). Biopharmaceuticals may have different compatibilities to materials used in the construction of product contact components, based on individual protein and formulation characteristics, as well as the leachables and extractables. Therefore, the impact of product contact components may need to be compared. Sub-visible particles induced by shear stress of two different syringe manufacturers were compared using RMM. These data are shown in Figure 3A.
1 mL syringes from two different manufacturers were used to induce shear-stress, as described in the Methods section. From the data in Figure 3A, the number of sub-visible particles formed by the syringe from manufacturer 1 (red bars) is much higher than the syringe from manufacturer 2 (blue bars). These data would suggest that the protein is more compatible with syringes from manufacturer 1 than those from manufacturer 2

|
The ability to distinguish silicone from protein aggregates based on buoyant mass is a significant benefit for the Archimedes RMM system. The positive mass data from the previous experiment is shown in Figure 3B, and demonstrates the presence of silicone oil from the two syringes tested. While protein aggregates produce a negative frequency shift, silicone which has a positive buoyant mass in water, produces a positive frequency shift (Figure 3C). This technology enables quantification of silicone oil content, which can have a detrimental impact on the protein itself, and / or an increase in immunogenicity following administration. From the data shown in Figure 3, the syringes from manufacturer 1 (red bars) introduces significantly higher levels of silicone oil into the protein sample, following repeated shear-stress. The increased levels of silicone oil may be the cause of the higher levels of protein aggregates detected with this syringe. However, the ability to detect and quantify silicone oil content provides a more detailed insight into the product, and therefore more knowledge concerning the relevant degradation pathways.
As recommended by the FDA, the ability to distinguish protein aggregates from non-protein material is an important aspect of monitoring and studying biopharmaceutical stability. This application note demonstrates the ability of the Archimedes system to quantify protein aggregates and silicone oil in response to shear-stress. Such data is greatly beneficial when understanding product stability, immunogenicity and bioprocessing impact. In addition, the ability to distinguish protein aggregates and silicone oil can enable more efficient resolution of product investigations. By quickly ruling in or out silicone oil as a potential root cause (3), efforts can be focused on finding the most likely causes. Importantly, the Archimedes is able to quantify protein particles in a size range that is currently poorly served by analytical instruments, between 0.2-5 µm, that has been identified as an important size range for understanding immunogenicity (1).
In conclusion, the Archimedes RMM system is able to provide highly valuable information about biopharmaceutical products, in terms of both formation of sub-visible particle formation and the presence of non-protein contaminants, such as silicone oil.
Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products (2013): FDA
Code of Federal Regulations; CFR21, Part211.65
Characterization and Quantitation of Aggregates and Particles in Interferon-β Products: Potential Links Between Product Quality Attributes and Immunogenicity (2013): J. Pharm. Sci. (102) p.915