Nanomedicines have the potential to revolutionize healthcare by prolonging the biological half-life of active ingredients, improving targeting, reducing toxicity and increasing bioavailability. In order to realize this promise, the pharmaceutical industry needs fit-for-purpose analytical methods that support cost-effective, large-scale production techniques for liposomal and lipid nanoparticle delivery vectors.
With years of experience in the development and manufacturing of liposomal and LNP formulations, Polymun Scientific is leading the way in transforming this promising technology into production reality.
Nanomedicines have the potential to revolutionize healthcare by prolonging the biological half-life of active ingredients, improving targeting, reducing toxicity and increasing bioavailability. In order to realize this promise, the pharmaceutical industry needs fit-for-purpose analytical methods that support cost-effective, large-scale production techniques for liposomal and lipid nanoparticle delivery vectors.
With years of experience in the development and manufacturing of liposomal and LNP formulations, Polymun Scientific is leading the way in transforming this promising technology into production reality.
![[Figure 1 CS211217-Nanomedicine-analytical-workflow-vaccine-production.png] Figure 1 CS211217-Nanomedicine-analytical-workflow-vaccine-production.png](https://dam.malvernpanalytical.com/4b79f95c-b898-4b58-9768-ae010093101a/Figure%201%20CS211217-Nanomedicine-analytical-workflow-vaccine-production_Original%20file.png)
Figure 1. Polymun scientists at work (the image courtesy of Polymun Scientific).
Until 2020, liposome and LNP formulations were a low volume, niche application. This changed when LNP–mRNA vaccines emerged as the most promising way to protect humanity from the novel coronavirus, SARS-CoV-2.
Contracted to produce the LNPs needed to deliver the BioNtech-Pfizer COVID-19 vaccine, the Polymun team had to increase production volumes in order to meet unprecedented demand from LNP vectors. To support the volumes required, the team had to develop robust manufacturing processes and analytical controls to support large-scale, GMP production processes.
Applying their unique know-how and cutting-edge technology, Polymun established new analytical methods to guide process development, control manufacturing processes, and confirm the batch-to-batch consistency and long-term stability of liposomal and LNP products.
Andreas Wagner, Head of Liposome Technology at Polymun Scientific states: “Our highly specialized scientific team found Malvern Panalytical’s Zetasizer DLS a key tool in Polymun’s analytical workflow”. The Zetasizer Ultra enables robust measurement of the size and size distribution of liposomal and LNP delivery vectors. These properties are critically important for ensuring the quality and stability of the final drug products, such as mRNA COVID-19 vaccines.
A narrow size distribution is necessary for the performance of liposomal and LNP products as it determines both targeting and transport characteristics. Particle size and polydispersity of the liposomal and LNP preparation are among the CQAs used to confirm that product quality is as expected, to detect sample instability, identify optimal process and formulation conditions, and to confirm batch-to-batch consistency.
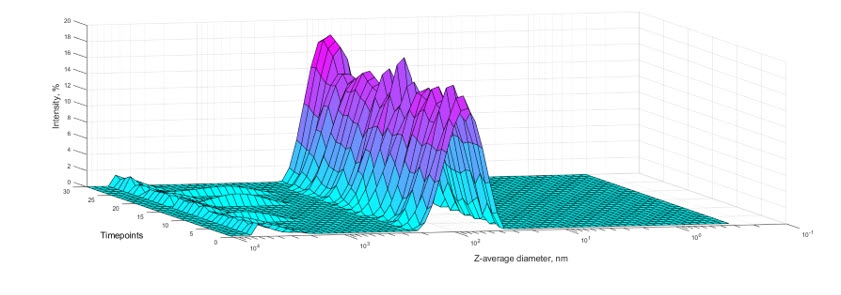
Figure 2. Time-dependent stability of an LNP preparation monitored through measurements of Z-average diameter of the sample with Zetasizer DLS Ultra.
Polymun’s expertise in liposome and LNP production is critical to companies developing therapeutics based on nano-delivery vectors. The production technology is tightly controlled and is suitable for a broad range of active substances such as oligonucleotides, small molecules, and mRNA.
The Malvern Panalytical team is proud to see how our analytical systems and services are making a vital contribution to the implementation of new scientific ideas, innovative processes and the production of medicines that are key to the fight against global health threats.