Application protocols for MicroCal Auto-iTC200
MicroCal Auto-iTC200 comes with a preloaded set of automation methods. This protocol describes the usage of the standard automation methods. All automation methods provided with the systems are summarized in Appendix A.
Method setup - overview
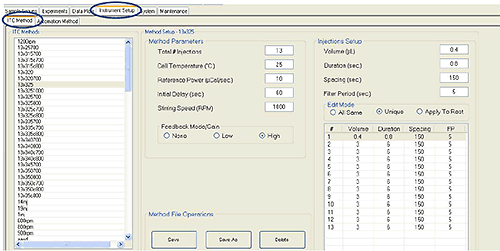
1. Select the Instrument Setup tab and the ITC Method subtab. Create and save a standard ITC protocol according to figure 1.
Figure 1. A standard ITC protocol with 12 * 3 μl injections.
|
- This protocol will work for most titrations. For some experiments it may be preferable to do 18 * 2 μl injections instead.
2. Select the Sample Groups tab and the Methods subtab and choose the appropriate automation method from the Automation Method drop-down menu. The default method Plates is a good starting point, but other methods may be more suitable depending on the application. See section Automation methods below to find an appropriate method.
Automation methods
Plates is the default automation method
- Protein and ligand are taken from a well plate
- This method washes the cell with 20% Contrad™ (14% Decon™) and rinses with water
- It holds protein in tubing for 5 minutes before loading the cell
- It washes the pipette with water, rinses with methanol and then dries with nitrogen gas
- ~1 hr 10 minutes/run (1hr 30 minutes for first run)
Plates2 is an alternative method to Plates
- Protein and ligand are taken from a well plate
- This method washes the cell with 20% Contrad™ (14% Decon™) and rinses with water
- The sample is first loaded into the cell to equilibrate at experimental temperature. The sample is then removed to help to remove air bubbles and then put back into the cell.
- It washes the pipette with water, rinses with methanol and then dries with nitrogen gas
- ~1 hr 10 minutes/run (1hr 30 minutes for first run)
Tubes is used for screening against target protein
- Tubes is exactly the same as Plates except that the cell material is taken from a 30 ml tube in the tube rack drawer (can hold five 30 ml tubes)
- ~1 hr 10 minutes/run (1hr 30 minutes for first run)
Tubes2 is an alternative method to Tubes
- Tubes2 is exactly the same as Plates2 except that the cell material is taken from a 30 ml tube in the tube rack drawer (can hold five 30 ml tubes)
- ~1 hr 10 minutes/run (1hr 30 minutes for first run)
Plates Quick/Tubes Quick for a clean system and non-sticky protein
- These methods wash the cell with water. If the protein is known not to stick to the ITC cell, then it can be cleaned with water only.
- When the instrument is clean and only one run is to be done
- Removing the detergent step will save the user ~15-20 minutes/run
- ~54 minutes/run (~1hr 12 minutes for first run)
Plates Syringe Clean/Tubes Syringe Clean for proteins in the syringe
- Both the cell and the syringe are washed with 20% Contrad™ (14% Decon™) and rinsed with water
- Use for protein:protein interactions and reverse titrations
- ~1 hr 10 minutes/run (~1hr 30 minutes for first run)
Plates Clean/Tubes Clean for aggressive cleaning
- The cell is cleaned by loading the cell with 20% Contrad™ (14% Decon™) and heating to 60 °C for 1 hr
- Use when Plates/Tubes are not enough i.e. they give noisy data and low baseline position
- Use for weekly maintenance
- ~2 hr 5 minutes/run (~2hr 25 minutes for first run)
Plates Prerinse/Tubes Prerinse are good for CaCl2 into EDTA experiments
- These methods prerinse the ITC cell with a solution of choice from a designated well
- Recommended for CaCl2 into EDTA tests to be able to prerinse the cell with EDTA
Plates Prerinse Syringe Clean/Tubes Prerinse Syringe Clean for aggressive cleaning
- Both the cell and the syringe are washed with 20% Contrad™ (14% Decon™) and rinsed with water
- These methods also prerinse the ITC cell with a solution of choice from a designated well
Continue Injections for concatenation
- This method removes 40 μl from the cell rather than emptying it
- It allows for the user to continue titrating in a second experiment
- Plates without the cell cleaning
Appendix A
MicroCal Auto-iTC200 automation methods
| Setup File | Cell Load | Cell Clean | Syringe Clean | Titrant Transfer Clean | Pre-rinse | Pre-equilibrate sample |
|---|
| Plates | P | D | W | W | N | Y (in tubing) |
| Plates Clean | P | D (extra) | W | W | N | Y (in tubing) |
| Plates Syringe Clean | P | D | D | D | N | Y (in tubing) |
| Plates Prerinse | P | D | W | W | Y | Y (in tubing) |
| Plates Prerinse Syringe Clean | P | D | D | D | Y | Y (in tubing) |
| Plates Quick | P | W | W | W | N | N |
| Tubes | T | D | W | W | N | Y (in tubing) |
| Tubes Clean | T | D (extra) | W | W | N | Y (in tubing) |
| Tubes Syringe Clean | T | D | D | D | N | Y (in tubing) |
| Tubes Prerinse | T | D | W | W | Y | Y (in tubing) |
| Tubes Prerinse Syringe Clean | T | D | D | D | Y | Y (in tubing) |
| Tubes Quick | T | W | W | W | N | N |
| Continue Injections | N/A | N/A | W | W | N | N |
P: Plate; T: Tube; D: Detergent; W: Water; Y: Yes; N: No
