Thin film XRD was employed for qualitative analysis of the chemical and biological properties of biomedical, electrosprayed calcium phosphate (CaP) coatings. XRD analysis provided in-depth examination of the relationship between processing conditions and the chemical nature of the deposited coatings. In addition, the reactivity of electrosprayed CaP coatings was monitored under in vitro and in vivo testing conditions. XRD characterization revealed that bioactivity and degradation behavior of bioceramic coatings are strongly dependent on crystallographic properties.
In the biomedical field, coatings are frequently applied to the surface of metallic dental and orthopedic implants to improve biological performance. Calcium phosphate (CaP) ceramics, due to a similarity to the inorganic component of bone and teeth, are commonly used for this purpose.
In the biomedical field, coatings are frequently applied to the surface of metallic dental and orthopedic implants to improve biological performance [1]. Calcium phosphate (CaP) ceramics, due to a similarity to the inorganic component of bone and teeth, are commonly used for this purpose.
CaP materials are bioactive ceramics, enabling the implant material to bond with the surrounding osseous tissue. This results in a strong interface between bone and the implant surface [2]. Generally, bioactivity is characterized by the formation of an intermediate CaP layer at this interface [3]. The capacity of biomaterial surfaces to form an intermediate CaP layer is strongly related to the physicochemical properties of the implant material surface. Characteristics such as chemical composition, crystal phase and crystallinity all play a key role [4]. These properties determine the chemical stability of bioceramic coatings in aqueous solutions, and thereby their reactivity in physiological environments.
A recently developed Electrostatic Spray Deposition (ESD) technique offers a novel method for applying bioceramic materials. ESD enables the controlled fabrication of coatings with a wide variety of chemical and morphological properties [5, 6].
ESD involves the generation of an ‘electrospray’ of charged, micrometer- sized droplets. This is accomplished by electrostatic atomization of solutions containing inorganic salts. Spray droplets are subsequently attracted by a grounded and heated substrate as a result of an applied potential difference. After complete solvent evaporation, a thin layer is left on the substrate surface.
XRD is a powerful tool for the characterization of the chemical nature of bioceramic coatings and bulk bioceramics. XRD was used to evaluate the crystallographic properties of biomedical ESD-coatings, in relation to their final biological behavior.
A commercially available ESD system (Advanced Surface Technology, Bleiswijk, the Netherlands) was used to deposit CaP coatings onto pure titanium (Ti) implants. Several deposition factors were varied to study the influence of processing conditions on deposited CaP coatings. Deposition parameters investigated were: precursor liquid composition; nozzle to substrate distance; precursor liquid flow rate; deposition temperature; and spraying nozzle geometry.
After deposition, all CaP-coated Ti substrates were heat-treated for two hours in air at 700 °C to crystallize the thin ceramic films. Biological performance of electrosprayed CaP coatings was evaluated using established in vitro and in vivo testing methods. Reactivity of CaP coatings was determined with the classical in vitro test - by immersing CaP-coated Ti substrates in Simulated Body Fluid (SBF). For in vivo testing, CaP coatings were evaluated after implantation in goats.
In addition to XRD, Fourier-Transform Infrared Spectrometry (FTIR) and energy dispersive X-ray fluorescence spectroscopy (EDXRF) were also employed to investigate respectively the molecular structure and elemental composition of the deposited coating.
Figure 1: Experimental setup of ESD system in downwards-facing configuration
For the thin film measurements at a grazing angle of incidence a programmable divergence slit set at a fixed divergence angle, a parallel plate collimator with flat- crystal monochromator and a point detector were used. Soller slits (0.04 rad) on both sides limited the axial divergence of the X-ray beam.
Thin film analysis of CaP coatings can be performed by XRD analysis with a Malvern Panalytical Empyrean system with Cu Kα radiation in grazing incidence configuration based on a parallel beam geometry. Soller slits (0.04 rad) and a 10 mm mask are used to limit the axial divergence of the beam. In the diffracted beam path a parallel plate collimator, a flat crystal monochromator and a point detector can be used.
Chemical analysis
Coating samples were analyzed by fixing the angle of incidence ω at 2.5° with the detector scanning set between 20° and 50° 2θ. Step size was 0.020° 2θ/s, scanning speed 0.010° 2θ/s, and sample time 2 seconds per step.
Biological analysis
Coating samples were analyzed by fixing the angle of incidence ω at 2.5° with the detector scanning set with a step size of 0.010° 2θ, scanning speed 0.010° 2θ/s, and a sample time of 1 second per step. Scan range was between 8° and 37° 2θ (in vitro) or 24° and 37° 2θ (in vivo).
Chemical analysis
XRD and FTIR analyses revealed that, depending on processing conditions, various crystal phases were synthesized after heat treatment, including: carbonate apatite, α/β- tricalcium phosphate (TCP), calcium metaphosphate, calcium pyrophosphate and monetite. An example of this relationship is shown in Figure 2.
Precursor acidity
Various CaP crystal phases were obtained, depending on the acidity of the precursor solutions. With the most acidic precursor solution (2% HNO3), β-pyrophosphate was the main crystal phase, with diffraction peaks at 31.2° 2θ and 34.8° 2θ indicating whitlockite. Less acidic precursor solutions (1% HNO3) showed mainly deposited whitlockite, with a small peak at 31.8° 2θ which could be minor contamination.
The diffractogram of the coating deposited using 0.1% HNO3 corresponded to a poorly crystalline apatite phase. While no addition of HNO3 results similarly in an apatitic crystal phase, but with calcium oxide as an impurity phase.
Figure 2: XRD patterns of electrosprayed CaP coatings prepared using different amounts of HNO3 addition;
(A) 2 vol% of HNO3 ;
(B) 1 vol% of HNO3 ;
(C) 0.1 vol% of HNO3 ;
(D) 0 vol% of HNO3
*= β-pyrophosphate(Ca2P2O7 );
˄ = whitlockite(Ca3(PO4 )2);
+ = apatite (Ca5(PO4)3 X);
# = rutile (TiO2 );
x = calcium oxide (CaO);
0 = titanium
Other deposition factors
Based on investigation of other deposition factors [7], the coating formation of the various crystal phases has been suggested to be the result of the following acid-base reaction:
2 HPO42- + CO32- → 2 PO43- + CO2 + H2O
Biological analysis in vitro
For the biological evaluation of electrosprayed CaP coatings, carbonated hydroxyapatite (CHA) was studied for its crystallographical similarity to bone mineral. For its high degree of degradability, β-TCP coatings were also subject to biological assessment.
Within the first week of immersion in SBF, highly bioactive behavior was observed for crystalline apatitic coatings, resulting in the rapid formation of a homogeneous precipitation layer of densely packed CaP flakes.
FTIR and XRD analyses revealed that all precipitation layers initially consisted of octacalcium phosphate (OCP) (see Figure 3a); and Ca/P ratios of OCP- covered apatitic coatings had decreased as a result of the low Ca/P ratio of OCP. Over time, OCP precipitation layers became more apatitic in nature. This was shown by:
i) the gradual loss of OCP low-angle reflection peaks (XRD, Figure 3a),
ii) the corresponding loss of degeneracy in the ν4 phosphate absorption domain,
iii) an increase in Ca/P ratio (EDXRF).
The gradual phase transformation from OCP to more basic apatitic CaP phases is a well-known phenomenon. It has been observed in vitro and in vivo after long periods of immersion [8]. Heterogeneous calcification was observed on β-TCP coated substrates for immersion periods of up to 8 weeks (Figure 4). This indicates that β-TCP coatings exhibit a poor calcification inducing ability in SBF, compared to crystalline apatitic coatings.
Figure 3: XRD patterns of CHA
[a] and β-TCP
[b] coatings after 0, 2 and 12 weeks of soaking in SBF
+ = apatite (Ca5(PO4)3X);
* = OCP(Ca H (PO ) .5H O);
^ = β-TCP (Ca3(PO4)2);
0 = titanium (Ti);
# = rutile (TiO2)
CHA coatings were heat-treated at 700 °C and consequently exhibited a lower dissolution rate than β-TCP. This was shown by the maintenance of typical apatitic reflection peaks up to 12 weeks after implantation.
Whereas β-TCP coatings had degraded completely after 8 weeks, despite similar heat treatment.
A comparable implantation study was performed in a bony implant site (a goat’s knee) using identical, porous CaP coatings electrosprayed onto Ti dental screws. Results showed that the best biological response in terms of bone contact was observed for stable CHA coatings. This suggests that long-term stability of electrosprayed, porous CHA coatings is beneficial for the desired integration of ESD-coated implants into bone tissue.
Biological analysis in vivo
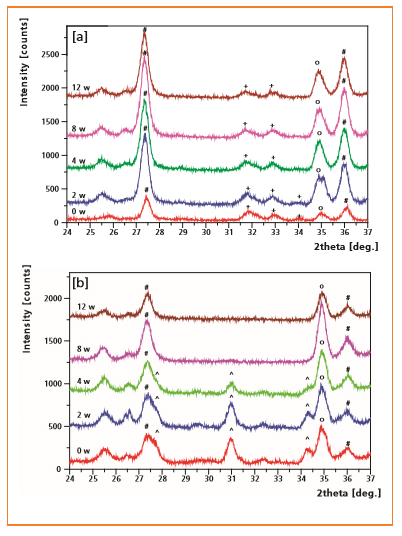
XRD and scanning electron microscopy revealed gradual degradation for all ESD-coatings after subcutaneous implantation into goats. This was indicated by a continuous decrease in intensity of CaP reflection peaks (Figure 5).
Figure 4: Tilted (45°) scanning electron micrograph of β-TCP after 2 weeks of soaking in SBF
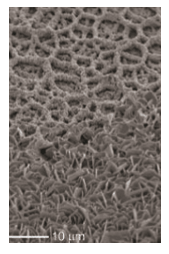
Figure 5: XRD patterns of CHA
[a] and β-TCP
[b] coatings after 0, 2, 4, 8 and 12 weeks of subcutaneous implantation
+ = apatite (Ca5(PO4)3X);
^ = β-TCP (Ca3(PO4)2);
0 = titanium (Ti);
# = rutile (TiO2)
Thin film XRD, in combination with techniques such as FTIR and EDXRF, allows the qualitative and semi-quantitative analysis of the chemical and biological characteristics of CaP coatings.
Various CaP phases and phase mixtures were identified using XRD. This information improves understanding of the physicochemical formation mechanism of electrosprayed CaP coatings. Moreover, XRD was demonstrated to be a powerful tool for evaluating the biological behavior of electrosprayed CaP coatings, depending on their physicochemical nature.
Mineralization and degradation phenomena can be monitored closely for in vitro and in vivo studies using this versatile analytical technique, thereby offering unique insights into the fundamental relationship between artificial, man-made biomaterials and living tissue.