Drugs based on proteins, or biopharmaceuticals are useful for the treatment of a wide range of illnesses and form one of the fastest-growing areas in medical research. As these products are used in vivo to treat disease, they are subject to extremely high regulatory burdens and must be able to demonstrate that they are effective but also safe.
The stability and aggregate level that these biologics contain is of primary concern as they influence the efficacy and immunogenicity of the final product. Detailed information on these parameters needs to be provided to help scientists drive development in the direction that produces a high quality and reliable product. Importantly it is also essential information for regulators to qualify and allow the release of the product.
Changes in FDA regulations allowed the development of biosimilars which have the same function as the innovator but are significantly cheaper. Biosimilars have shortened licensing pathways when compared to innovator materials if they can be shown to be biologically similar to already licensed products. For a product to be considered as biosimilar it must undergo detailed analytical studies demonstrating that it is highly similar to the innovator and include information to show that it is expected to produce the same clinical response as the innovator in any given patient.
A successful biosimilar program has comparative analytical data as its foundation. As part of the analytical study, the FDA requires the use of state-of-the-art technology to compare higher-order structures, including aggregation, of the biosimilars with the innovator in addition to any formulation effects on purity, stability, product and process-related impurities.
Log in or register for free to read more.
Drugs based on proteins, or biopharmaceuticals are useful for the treatment of a wide range of illnesses and form one of the fastest-growing areas in medical research. As these products are used in vivo to treat disease, they are subject to extremely high regulatory burdens and must be able to demonstrate that they are effective but also safe.
The stability and aggregate level that these biologics contain is of primary concern as they influence the efficacy and immunogenicity of the final product. Detailed information on these parameters needs to be provided to help scientists drive development in the direction that produces a high quality and reliable product. Importantly it is also essential information for regulators to qualify and allow the release of the product.
Changes in FDA regulations allowed the development of biosimilars which have the same function as the innovator but are significantly cheaper. Biosimilars have shortened licensing pathways when compared to innovator materials if they can be shown to be biologically similar to already licensed products. For a product to be considered as biosimilar it must undergo detailed analytical studies demonstrating that it is highly similar to the innovator and include information to show that it is expected to produce the same clinical response as the innovator in any given patient.
A successful biosimilar program has comparative analytical data as its foundation. As part of the analytical study, the FDA requires the use of state-of-the-art technology to compare higher-order structures, including aggregation, of the biosimilars with the innovator in addition to any formulation effects on purity, stability, product and process-related impurities.
A key tool in demonstrating biosimilarity is multi-detection SEC. The OMNISEC system from Malvern Panalytical is the world's most advanced multi-detection SEC system. The detector module, OMNISEC REVEAL, contains a refractive index (RI) and UV/VIS PDA detector to measure concentration, a right angle and low angle light scattering (RALS/LALS) or multi-angle light scattering (MALS) detector to measure absolute molecular weight and an online differential viscometer (Visc) to measure intrinsic viscosity and size.

Figure 1. OMNISEC multi-detection SEC system
The OMNISEC system allows the absolute molecular weight, oligomeric state, purity and size of a sample to be measured in a single injection. This application note presents a series of results obtained using the OMNISEC for innovators and biosimilar candidates as well as discusses the data provided for a biosimilar submission for approval by the United States Food and Drug Administration (USFDA).
Bevacizumab (Avastin®) is an angiogenesis inhibitor that functions by preventing the growth of blood vessels in cancerous tumors by inhibiting VEGF-A. Treatment with Bevacizumab has been shown to be beneficial for the treatment of colorectal as well as other forms of cancer.
One of the first steps in proving biosimilarity is properly characterizing the innovator material. The chromatogram below is typical for the Bevacizumab innovator.
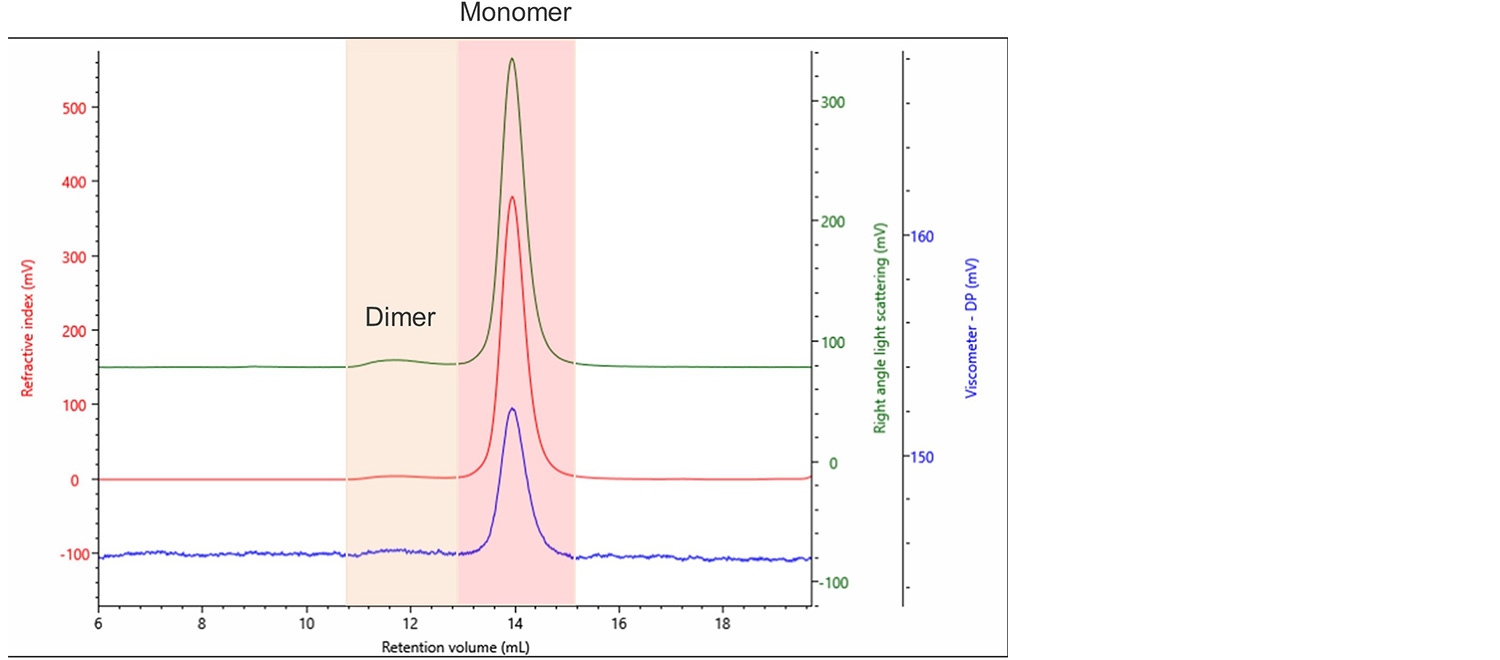
Figure 2. Typical chromatogram of bevacizumab produced by the OMNISEC system. Refractive index (RI) (Red), right angle light scattering (RALS) (Green), viscometer (Blue).
Two clear populations can be seen. They were identified as the monomer and dimer of bevacizumab, as highlighted in Figure 2. Multiple injections of this innovator were performed to obtain statistically robust results, as shown in Table 1.
| Mw (g/mol) | IV (dL/g) | Rh (nm) | Frac. of sample (%) | ||
|---|---|---|---|---|---|
| Peak 1 | Mean | 290,600 | 2.6 | ||
| Percent RSD | 0.9 | 3.8 | |||
| Peak 2 | Mean | 154,600 | 0.046 | 4.8 | 97.4 |
| Percent RSD | 0.11 | 4.03 | 1.33 | 0.099 | |
| Table 1. Quantitative results for bevacizumab innovator. | |||||
The sample was analyzed with two sets of limits. The first is indicated as Peak 1. It is the less intense dimer peak that elutes from roughly 11.0 to 12.5 mL retention volume (RV). Whereas Peak 2 is the main peak which elutes from 13 to 15 mL RV. Percent RSD is the relative standard deviation expressed as a percentage. Mw is the absolute molecular weight, IV is the intrinsic viscosity, Rh is the hydrodynamic radius and frac. of sample is the fraction of the sample that the peak represents. As can be seen, 97.4% of the innovator is monomeric with 2.6% dimer. The Mws of the monomer and dimer were accurately calculated, and no high molecular weight aggregates were seen.
Once the innovator material has been characterized a detailed comparison with the biosimilar needs to be performed. The first step in this process is comparing overlays of the raw data collected from the different detector channels. In this case, the biosimilar and innovator are in similar buffers, at similar concentrations without any external stress imparted on them. In Figure 3 an overlay of the RI and RALS detectors shows that both the bevacizumab innovator and biosimilar have very similar chromatography.
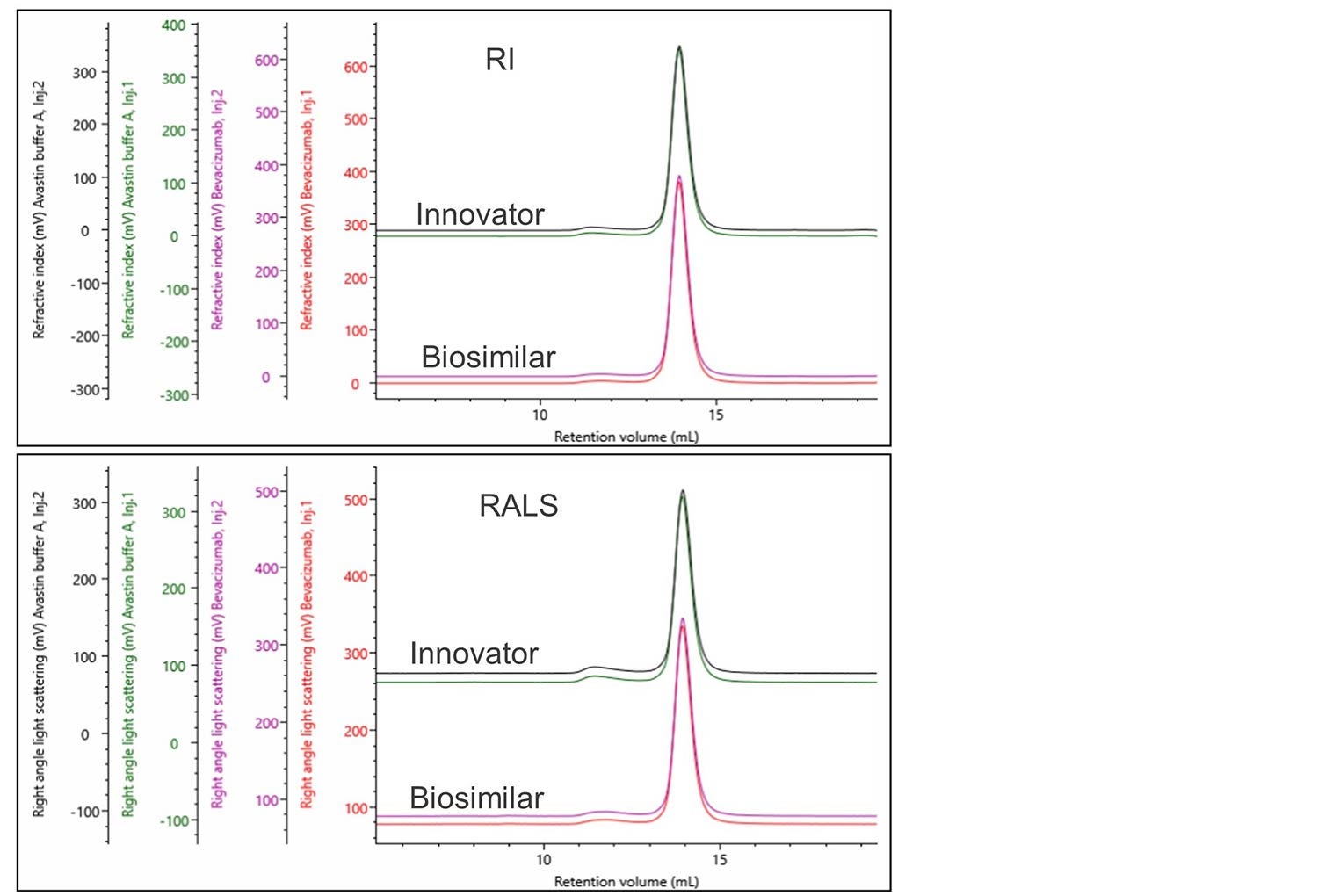
Figure 3. RI and RALS overlay of bevacizumab innovator and biosimilar.
Having confirmed the raw data is similar, a full analysis of the biosimilar was performed and the results in Table 2 were produced.
| Mw (g/mol) | IV (dL/g) | Rh (nm) | Frac. of sample (%) | ||
|---|---|---|---|---|---|
| Peak 1 | Innovator | 290,600 | 2.6 | ||
| Biosimilar | 289,700 | 3.4 | |||
| Peak 2 | Innovator | 154,600 | 0.046 | 4.8 | 97.4 |
| Biosimilar | 155,400 | 0.049 | 4.9 | 96.6 | |
| Table 2. Comparison of the quantitative results for bevacizumab innovator and biosimilar. | |||||
A small difference of 0.8% is observed between the monomer fraction of the innovator and the biosimilar, whereas the rest of the data is similar. This difference seems small but might need to be investigated further.
During the development of a biosimilar appropriate formulation conditions also need to be developed. The same process can be used to assess these conditions. Figure 4 shows the overlay of the innovator and a biosimilar produced using a different formulation buffer.
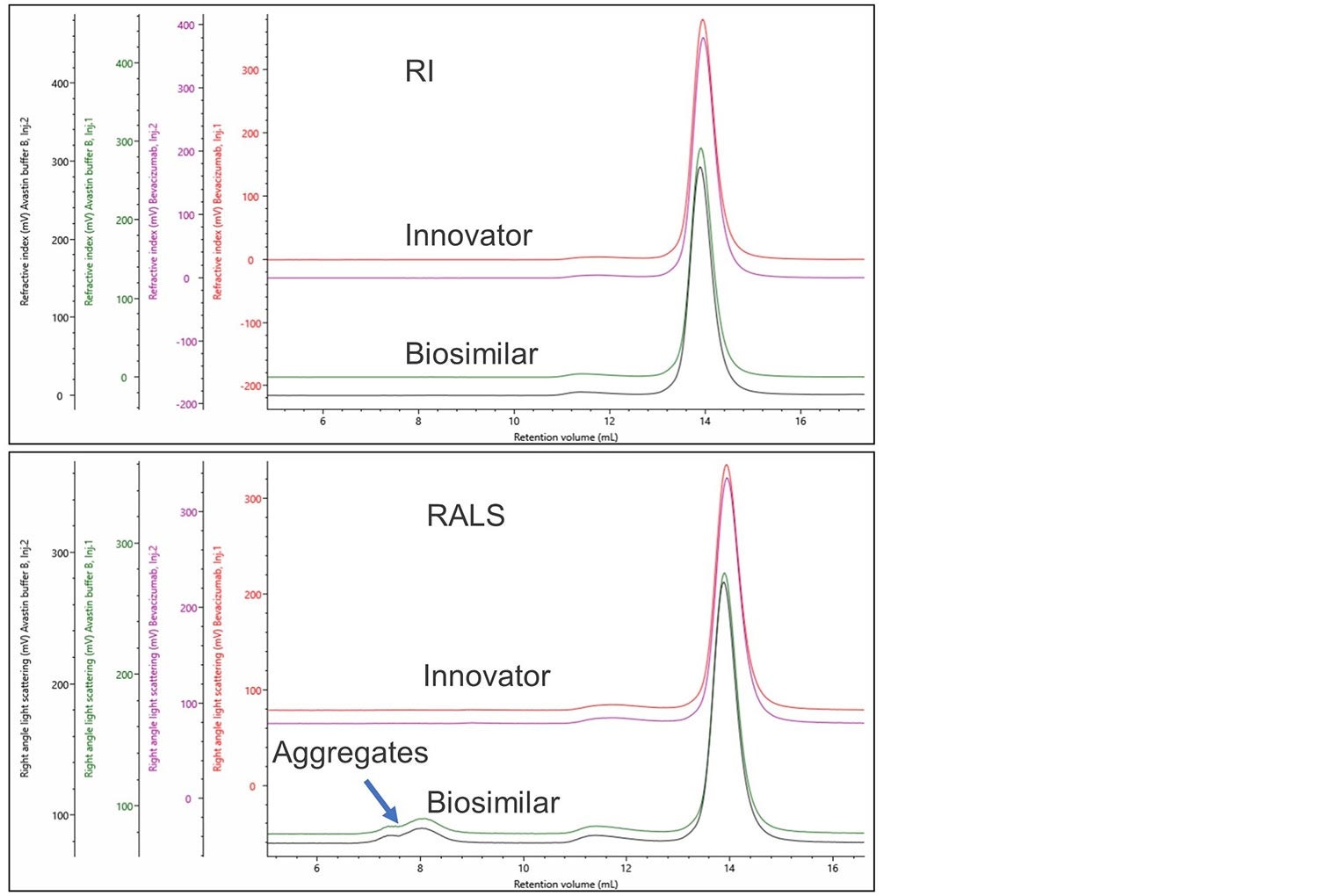
Figure 4. RI and RALS overlay of bevacizumab innovator and biosimilar in an alternative formulation.
Using a single concentration detector, no difference can be seen between these two formulations, the inclusion of a light scattering detector clearly shows the presence of high molecular weights aggregates in the biosimilar in the alternative formulation. This demonstrates it is essential to use multi-detection SEC when developing biosimilars and investigating formulations. This methodology can be used to quantify and direct improvements in the production or handling processes of the biosimilar.
This process can be applied to other biosimilars and monoclonal antibodies. Denosumab (Prolia® and Xgeva®) is used to reduce the risk of broken bones in people with osteoporosis by inhibiting the cells that break down bone (osteoclasts).
In this example, the innovator and biosimilar for denosumab have been incubated at 30 oC for an extended period. Samples were treated in an identical manner in order to observe if the two samples are able to withstand stress similarly. Once again, overlays of the different detector signals are used to monitor any effects of this stress.
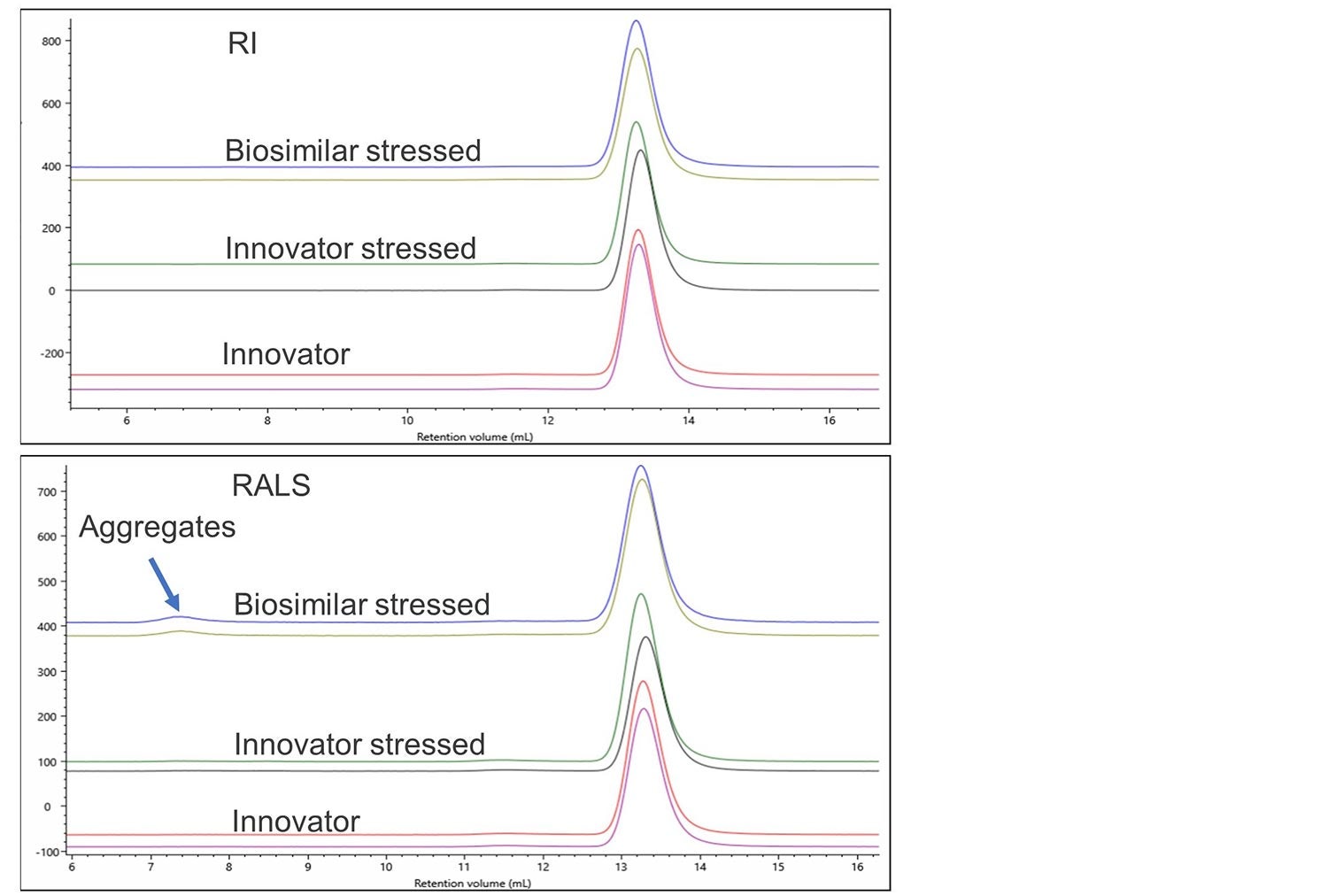
Figure 5. RI and RALS overlay of denosumab innovator, innovator stressed and biosimilar stressed.
Again, no differences can easily be observed using a single concentration detector. The light scattering detector shows significant aggregation in the stressed biosimilar and a small amount of aggregation in the stressed Innovator. Figure 6 shows a zoomed-in view of this aggregation on the RALS detector.
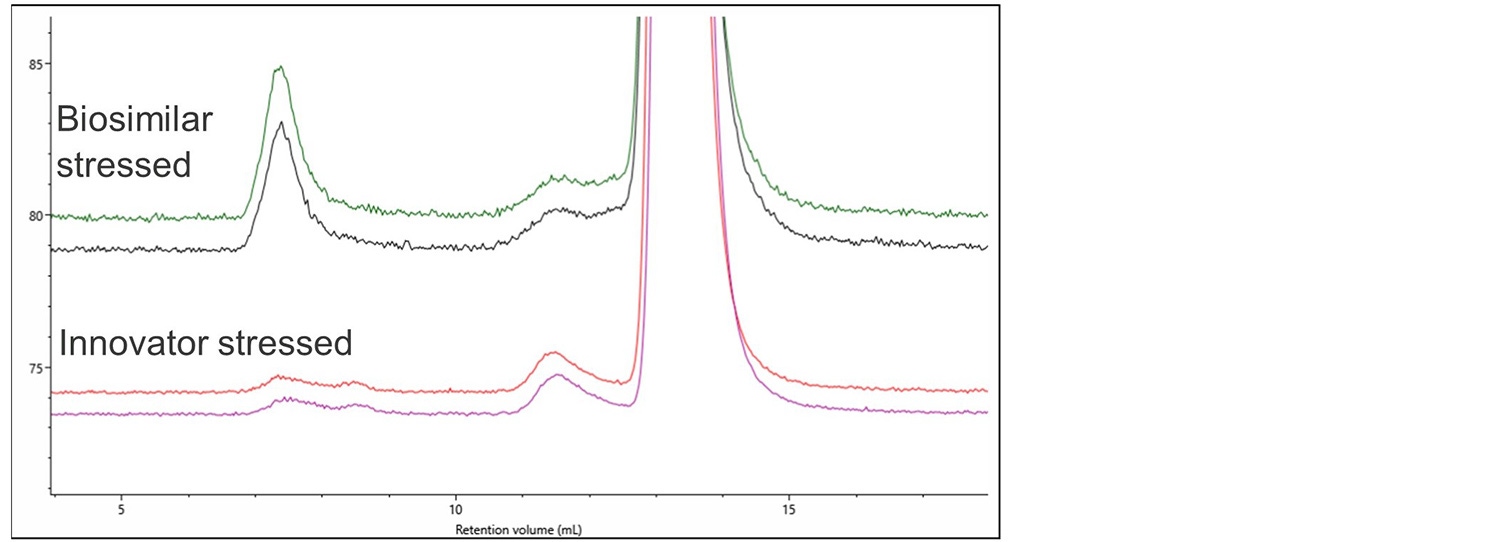
Figure 6. Magnification of the RALS signal showing aggregation in the biosimilar and innovator stressed samples.
As well as the more extensive aggregation in the biosimilar there is also a clear difference in the populations present, with the innovator showing 2 aggregate populations at 7 mL RV while the biosimilar has 1 larger aggregate.
In addition, the innovator has significantly more monomers present than the biosimilar. Analysis showed that the biosimilar contained 98.6% monomer, 1.3% dimer and 0.1% aggregate whereas the Innovator contained 99.2% monomer, 0.8% dimer and negligible amounts of aggregate. These differences could indicate a different route to the formation of aggregates which could be significant to the pass or failure of this biosimilar.
The biosimilar market is not just focused on monoclonal antibodies but growth factors e.g. epoetin, hormones e.g. follitropin alfa and fusion proteins e.g. etanercept (https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf). For the latter, as of May 2019 two biosimilars have been accepted by the USFDA. Sandoz’ Erelzi was accepted in 2016. A highly informative document submitted for approval by the USFDA allows us to demonstrate how the OMNISEC would be used to do a section of the necessary characterization to illustrate biosimilarity (https://www.fda.gov/media/98962/download).
Firstly, the composition can be correlated to each of the monomer, multimeric and degraded products of etanercept (Figure 7). Detailed in Figure 7 is a multi-detection chromatogram of etanercept. The RI and RALS detectors are used to determine the molecular weight and concentration of each component, and the components are labeled with the equivalent etanercept products.
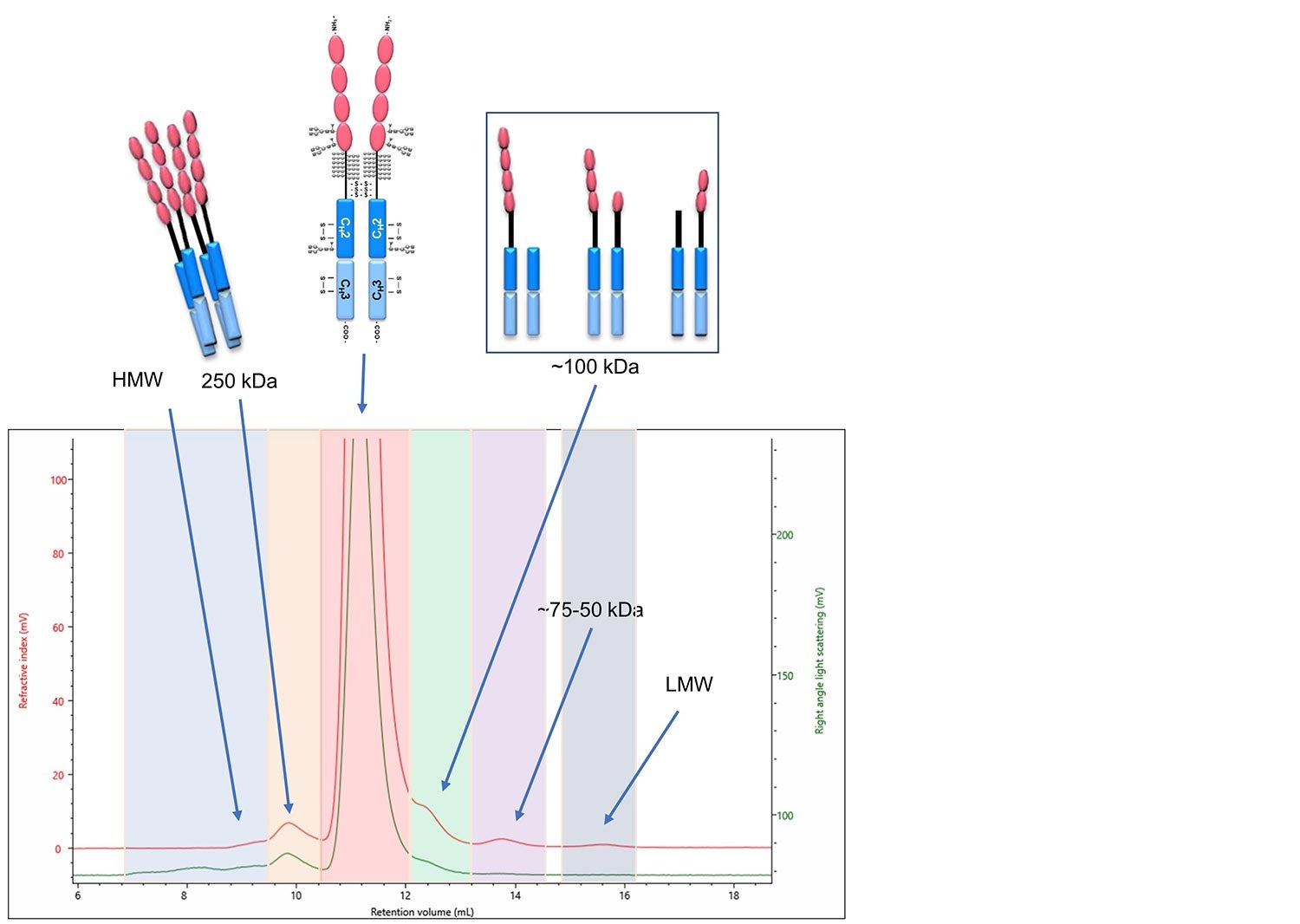
Figure 7. Multi-detection chromatogram of etanercept. The detectors shown are the RI in red and the RALS in green. Each section is indicated with the molecular weight as calculated using the OMNISEC software and is labeled with the equivalent etanercept product. Labels: HMW stands for high molecular weight and LMW stands for low molecular weight.
After the identification of each component in the biosimilar’s chromatogram, the composition can be compared to the innovator in order to indicate its biosimilarity. The first of which is the percentage of aggregated product, identified in the above figure as the two-colored sections labeled HMW and 250 kDa. They make up 2.8% of the above sample. One of the characteristics used by Sandoz to show their product, GP2015, as a biosimilar to the innovator product was by presenting that it had consistently less aggregated materials than the innovator. This is illustrated below in Figure 8, as extracted from United States Food and Drug Administration Arthritis Advisory Committee slides from July 13, 2016 (https://www.fda.gov/media/98962/download).
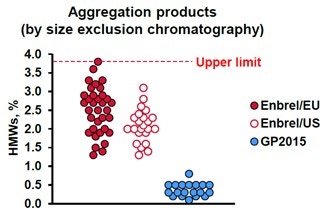
Figure 8. A plot of the percentage of aggregated products in various samples of the innovator: Enbrel/EU, Enbrel/US and the Sandoz’ submission for biosimilarity GP2015.
Additionally, the low molecular weight degradation products indicated in three distinct areas on the chromatogram above, labeled ~100 kDa, 75-60 kDa and LMW, were identified and quantified to be 3.4% of the sample. This is important for studying the stability of the product under the intended storage conditions. The fraction of degraded material in both the innovator and biosimilar can be measured over time to illustrate that the biosimilar has a similar stability behavior as the innovator. This was done in the study referenced above (overlaid graph Figure 9 b). Furthermore, in Figure 9 a, an overlay between two time points as analyzed by the OMNISEC system are shown. The two timepoints illustrate the sensitivity the OMNISEC has to identify the changes in the fraction of degraded product. The comparison shows how an Enbrel (etanercept innovator) sample is stored at 4 °C for 6 months has increased amounts of low molecular weight degradation products. The fraction of low molecular weight degradation products was observed to change from 3.4% to 4.4% over that period.
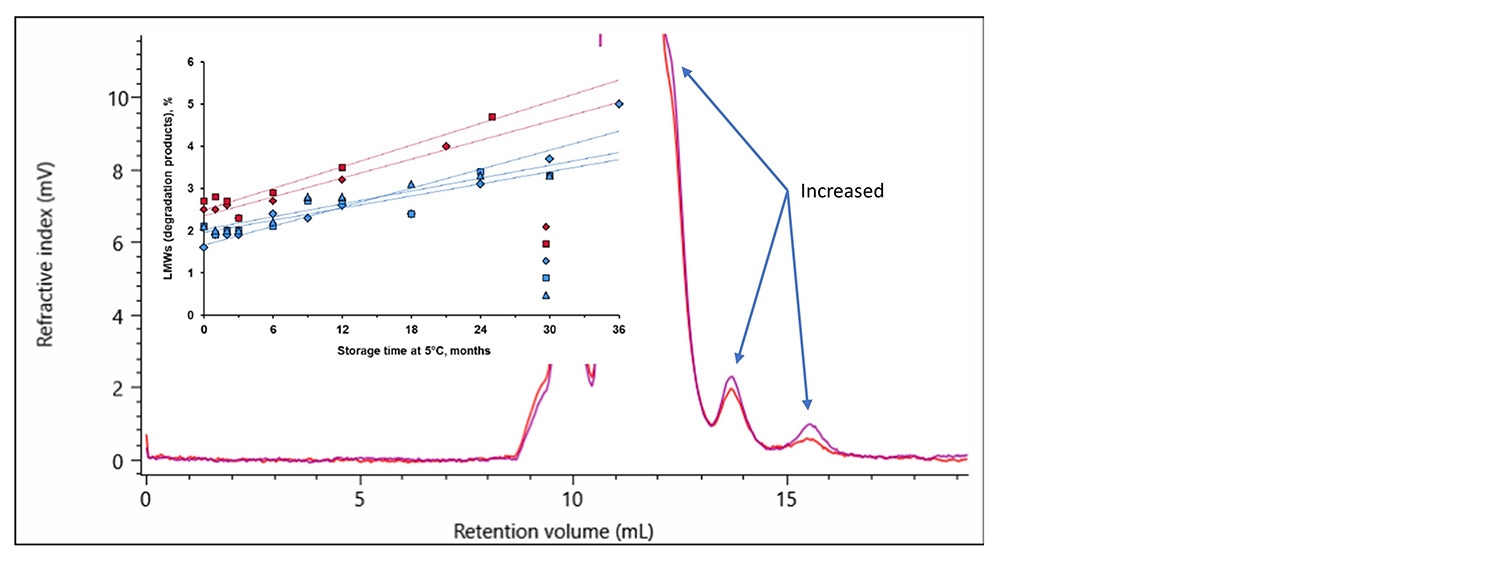
Figure 9. a. Overlay of RI chromatograms recorded for the etanercept innovator Enbrel produced by the OMNISEC system. The purple chromatogram is from a sample that had spent 6 months longer at 4 °C than the sample that produced the red chromatogram.
b. A figure extracted from the submission by Sandoz referenced above illustrating the percentage of low molecular weight degradation products in the innovator and their biosimilar as they aged at 5 °C.
Multi-detection SEC is an invaluable tool for the assessment of biosimilarity between biologicals. As has been demonstrated this technique allows the impact of different formulations and external stress conditions to be quantified. In addition, it has been shown that the OMNISEC can provide critical quality attributes as identified in previous biosimilarity submissions and has higher sensitivity to aggregate detection.