The aim of this application note is to provide advice as to how the principles of method validation may be applied to the characterization of nasal sprays using the Malvern Spraytec. It is based around the draft guidance provided by the FDA relating to the bioavailability and bioequivalence studies recommended for nasal sprays and nasal aerosols [1].
In recent years there has been growing interest in nasal spray devices for drug delivery. The advantages of using nasal spray devices for drug delivery include the ability to achieve drug absorption rates that are comparable to intravenous medication by non-invasive means. Size measurement is an essential part of the determination of bioavailability and bioequivalence in nasal sprays, as the spray droplet size relates to where deposition occurs in the nasal passages. Sizes in the range 20 to 150μm are desirable in order to achieve significant nasal deposition whilst minimizing the risk of droplets being carried through to the lungs.
In general, a validation study requires the evaluation of several characteristics. These can include accuracy, precision (including repeatability and intermediate precision), specificity, detection limit, quantitation limit, linearity, range and robustness. Not all of these characteristics need to be investigated for laser diffraction measurements. In particular, concepts such as accuracy, detection limit, quantitation limit, and linearity do not necessarily apply to particle size measurements [2, 3]. As such, the primary goal of users should be to test whether the chosen technique for droplet size analysis is appropriate for their product. The repeatability and intermediate precision of the proposed method should then be assessed.
The Spraytec is a laser diffraction instrument. It is not the aim of this application note to cover the validation of the technology. There are many sources of information available which describe the theory behind the technology and analysis involved in laser diffraction measurements, including ISO 13320 [4], USP<429>, EP 2.9.31 and NIST guides [5].
Following the procedures outlined in this note will not guarantee that a regulatory body will approve a method. The aim of this note is to provide a guide to the minimum requirements of a validation study. Any additional studies would therefore strengthen an application.
Before considering the parameters which need to be investigated it is important to ensure that the validation study is carried out on validated instrument running validated software.
The instrument must conform to the manufacturers Installation Qualification (IQ). In addition, the manufacturers Operational Qualification (OQ) should be carried out at least yearly. The software should be tested, for example by carrying out numerical validation against a peer package (e.g. Excel), and be compliant with 21CFR Part 11.
The variables which should be considered in order to validate a method for nasal spray droplet sizing by laser diffraction include: precision, sampling, sample preparation, range, specificity, and robustness. Although these parameters were discussed in the previous application note, a different approach will have to be taken to validating a method for nasal sprays.
Laser diffraction measurements allow different spray parameters, such as the Dv10, Dv50, Dv90 and laser transmission, to be measured as a function of time for each nasal spray actuation. This allows a profile of how these parameters vary with time to be determined. This profile is described in three phases, which are defined by the FDA according to the variation in the laser light transmission (or obscuration) [6].
First, the formation phase is observed, where the liquid begins to flow through the nozzle. The spray is unstable in this phase, producing large droplets, and the transmission remains high. As the flow increases, the transmission decreases sharply, as does the particle size, until a region of stable transmission is reached. This middle section is known as the fully developed phase and is the most important phase to consider as part of regulatory testing as it is the only phase within which reproducible measurements can be achieved. Finally, as the liquid flow rate through the nozzle decreases, the particle size increases rapidly as well as the transmission of the laser. This region defines the dissipation phase, where atomization of the liquid is again poorly controlled.
As it is results from the fully developed phase that are of interest to the regulatory body, the first task must be to determine the start and end point of the stable phase. This will allow the particle size to be determined from this phase only, and any subsequent results discussed in this application note will be taken from the fully developed phase only.
To determine the length of the fully developed phase we must look at the particle size history and the transmission profile obtained for actuation of the nasal spray. It is recommended that the transmission profile for the whole event should be determined at two distances from the nozzle and for multiple actuations. The method by which the fully developed phase is defined for each profile must then be explained (i.e. whether it is taken from the average a number of measurements, or a value from a single measurement).
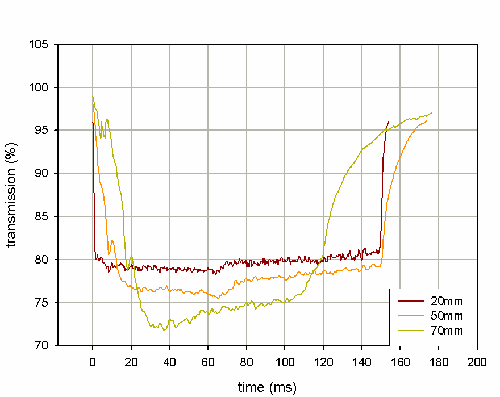
Within the Spraytec software, it is possible to create an ensemble average for a set of repeat actuations - this averaging method produces a size history profile where the mean of each size distribution parameter is calculated for same time point across multiple actuation events. The transmission profiles shown in figure 1 represent ensemble averages calculated for 3 repeat actuations at each of the stated measurement distances. These results demonstrate how the length of the stable phase is dependent on the distance at which the spray is measured.
|
|
Evaluating the transmission profile over the range of distances to be measured allows robust values for the beginning and end of the stable phase to be determined. For example, if the spray was to be measured at 20mm and 50mm, the stable phase could be defined as being between 20ms to 140ms for all measurements. The ensemble average function also calculates the standard deviation of each parameter at every time point. This function can be used to assess the variability of the particle size at each time point in the spray event and further aid in the determination of the position of the stable phase.
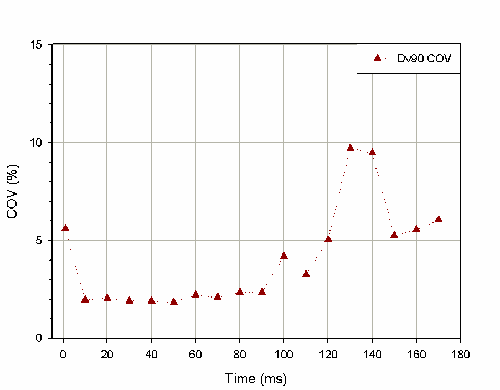
It is also important to consider how the time period selected for the stable phase average affects the reproducibility of the reported particle size distribution. Figure 2 shows how the coefficient of variation for stable phase Dv(90) changes as a function of the stable phase averaging period for 6 actuation of a pump. Selecting an averaging window of between 10msec and 90msec at the center of the actuation profile yields reproducible results. Selecting too long a time period causes the coefficient of variation to increase, as measurements from either the formation or dissipation phase are included as part of the stable phase average. The coefficient of variation is also high if only 1 record at the center of the profile is chosen to represent the stable phase.
Sampling of the material under test is one of the greatest challenges of particulate science. A sample is defined as a portion of material intended to be representative of the whole [5]. In the case of sprays, there is little control over how the formulation is metered by the pump, other than to ensure that the device is consistently shaken or primed before measurement. The most significant variable which affects the sampling of
|
sprays is the position of the spray nozzle relative to the laser beam.
Although each device will be different, in general the spray plume expands as the distance from the nozzle increases. Therefore, if the spray is measured at increasing distances from the nozzle, the laser beam will sample a smaller portion of the spray plume. It will then depend on the particular atomizer as to how this affects the particle size measured by the Spraytec. It is often the case that larger particles are forced to the outside of the plume and hence at greater distances the measured particle size is reduced. This sampling issue is recognized by the FDA in its draft guidance document [1]. It is recommended that measurements should be carried out at two distances in the range 20mm to 70mm and separated by 30mm. This ensures that any effects caused by sampling can be quantified.
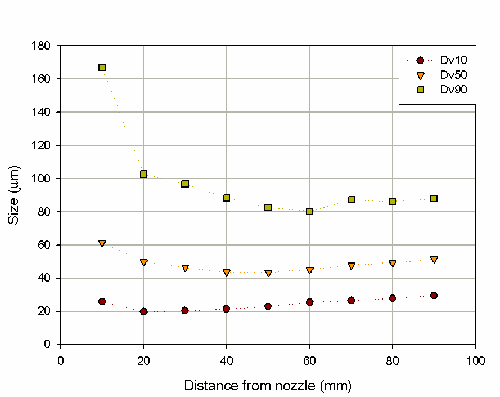
Here, the affect of distance, and therefore sampling, on the measured particle size has been investigated by measuring six repeat actuations at distances ranging from 10mm to 90mm from the center of the Spraytec measurement zone (figure 3). This shows that there is a decrease in particle size as the distance between the nozzle and the laser beam in increased. This sampling effect is significant as a coefficient of variance for the Dv50 of 11% was obtained over the range of measurements.
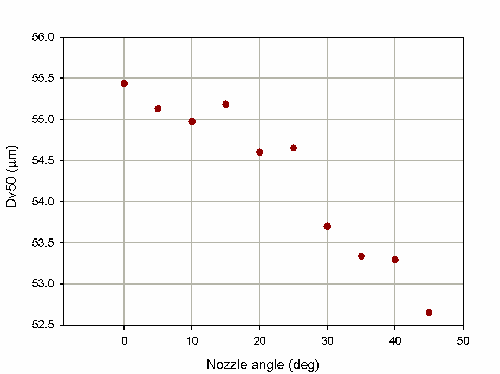
The Spraytec actuation station also has the option to angle the spray relative to the measurement zone (from 0 to 45deg). This allows the operation of the device to be investigated under more realistic conditions. For the nasal spray pump investigated in this note the measured particle size decreases as the angle is increased, as shown in figure 4. This tells us about the symmetry of the spray plume. Altering the angle has changed the portion of the spray plume which was sampled. In this case fewer large particles were sampled and the measured particle size was reduced. The dependence on the measurement angle is much weaker than that of the measurement distance. This is reflected in a %COV for the measurements of only 1.7% over the range of angles tested.
The effects of sampling in measurements of a nasal spray are caused by the position of the spray relative to the laser beam. Sampling effects can therefore be controlled easily within the Spraytec software, which allows the position and angle to be set in an SOP. Once the position and angle have been set, the measurement will not proceed until the nozzle is in the correct position.
In considering sample presentation, comparisons can be made to measurements of wet suspensions or dry powders where sample preparation is related to the state of dispersion of the sample. In the case of sprays the equivalent of sample preparation is the nature of the actuation profile used to actuate the nasal spray pump.
The use of an automated actuator is recommended in order to obtain reproducible results [1]. This is particularly important if a method is to be used by several analysts (or on different sites) as different people will actuate the device with a different force and velocity.
There are two different types of automated actuator available for use in conjunction with the Spraytec. Both provide reproducible actuation by controlling either the force applied to the device or the velocity at which the device is actuated. In this case a velocity controlled actuator has been used to investigate the effect of sample preparation on the particle size produced by a nasal spray, by varying the velocity and acceleration achieved during actuation.
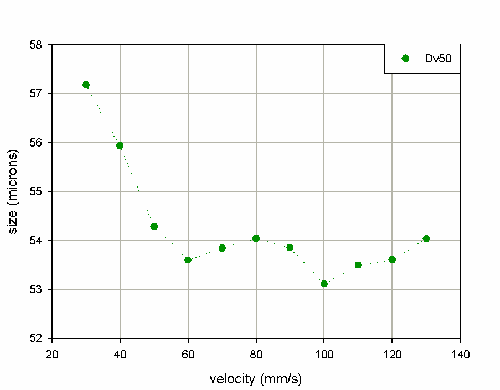
Figure 5 shows the variation in stable phase Dv50 as a function of velocity.
|
|
Initially, a decrease in particle size is observed as the velocity increases; this correlates with improved performance of the atomizer at higher liquid flow rates. Above 40mm/s the output of the pump becomes very reproducible, with a coefficient of variance of only 2.2% for the Dv50 for velocities between 40mm/s and 130mm/s. This suggests that measurements should be reproducible at higher velocities.
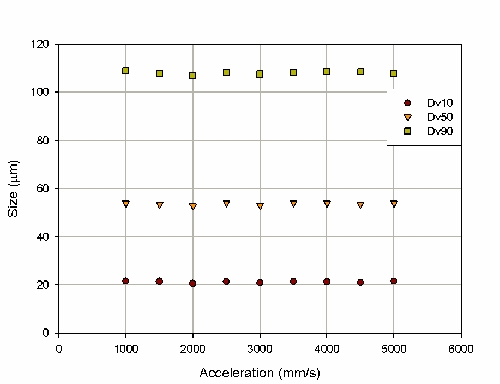
To determine the robustness of the velocity/acceleration controlled actuation, the effect of acceleration on the particle size was also investigated (figure 6). This produced no trend in the particle size and a random variation of 0.8% on the Dv50.
Although in this case the particle size was only very slightly dependent on velocity and independent of acceleration this may not be the case for all nasal spray devices. Of particular importance is the measurement at a range of velocities as this allows the actuation conditions which best represent the target patient group to be determined.
Specificity refers to the suitability of the technique to the material to be analyzed. Laser diffraction is particularly well suited to the measurement of nasal sprays due to the speed at which data can be acquired. High speed data acquisition is required in order to accurately characterize these devices, as the three phases of atomization generally occur in less than 400ms. This, in part, explains why laser diffraction is recommended for particle size analysis in the FDA draft guidance document [1]. However, it is also important to determine that the distribution of particle sizes produced by the device is within the range of the instrument. Most nasal spray devices are designed to produce particles between 20 μm and 150μm in size in order to optimize deposition in the nasal passages. This is well within the range of the Spraytec system's 300mm lens, which can measure from 0.1 to 1000μm. The size range can be confirmed by passing a glass slide through the spray plume and inspecting this under a microscope to ensure no droplets beyond the measurement range of the lens exist [6].
|
The robustness of an analytical technique describes its insensitivity to departures from underlying assumptions [2]. Robustness must be assessed before the reproducibility of the system is defined. In order to test the robustness of a method, intentional variations must be made to parameters which would normally be kept constant. The assumptions which will be tested in this application note relate to the horizontal position of the nasal spray between the transmitter and receiver modules of the Spraytec system and the position of the extraction hood.
The design of the Spraytec allows the transmitter and receiver units to be moved to different positions along the optical bench, in order to allow for the characterization of a wide range of sprays. The positions of the nasal spray actuator, the transmitter and the receiver are therefore not fixed and could be altered, either intentionally or unintentionally, between measurements. It is therefore important to determine the effect of a slight horizontal displacement on the measured particle size as this position can affect the accuracy of laser diffraction measurements. In particular, if the distance between the spray and the receiver optics is too large it may not be possible to accurately measure fine particles within the spray plume.
In order to investigate the effect of position on the measurement of a nasal spray, repeated measurements were carried out at increasing distances from the receiver optics. The first measurement was carried out at the smallest possible distance of 75mm (spray deposition on the optics occurred at distances smaller than this). The distance from the receiver was then increased in steps of 50mm until the maximum possible
|
distance of 475mm was achieved (limited by the length of the optical bench).
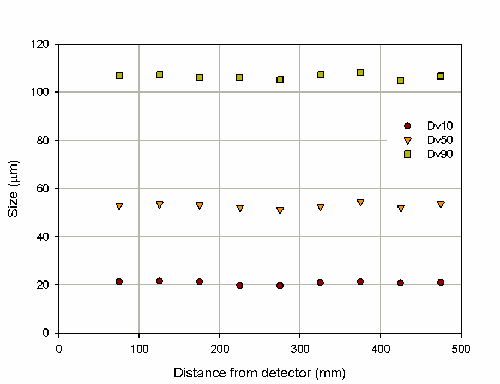
The variation in particle size with distance from the detector is shown in figure 7. This investigation has illustrated that for measurements of nasal sprays the Spraytec is not sensitive to variations in the position of the actuator. Practically this means that slight variations in the horizontal position will not affect results. The COV in the Dv50 over the range of distance investigated is 1.9%. However, it is recommended that the actuator is positioned approximately in the center of the optical bench between the detector and transmitter. This position will ensure that deposition on the optics will be minimized.
In the measurement of a nasal spray it is important to have an extraction system in place as this will minimize any measurement errors caused by velocity biasing. It is assumed that the droplets of all sizes travel across the beam at the same speed. In order to achieve a constant speed across the beam, the droplets need to be subject to a constant force. Therefore the force applied to the droplets from the device must be balanced by the extraction system; otherwise the droplets will slow down as they approach the measurement zone. Smaller droplet tend to slow down more quickly compared to coarse droplets and will consequently spend a longer time in the measurement zone if no extraction is used. This would have the effect of biasing the measured size distribution due to the over-sampling of the fine particle fraction.
The effectiveness of the extraction system will depend on its position relative to the spray nozzle. If the distance between the nozzle and the hood is increased then the suction acting on the droplets in the laser beam will be reduced. The aim of this section of this application note is to determine at what distance this reduction in extraction power will begin to affect the measurement. Measurements were carried out at increasing distances from 33mm to 273mm above the nozzle. A measurement was also carried out with the extraction system turned off in order to determine the distance at which the extraction system no longer had any effect of the spray plume velocity.
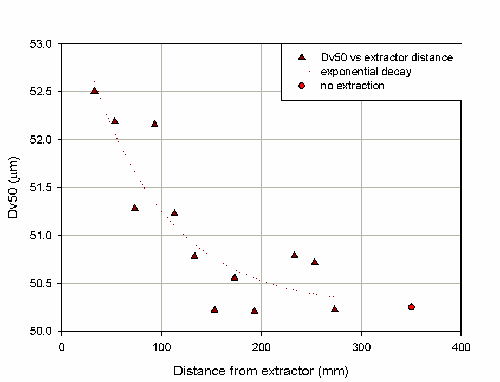
Figure 8 shows that there is a decrease in particle size as the distance between the nozzle and the extraction hood is increased. This is indicative of the over-sampling of fine material due to velocity biasing. Comparing these measurements to the result obtained with no extraction indicates that at distances above approximately 200mm the extraction is having little effect. The COV on the Dv50 of measurements made with the extraction system at a range of distance and switched off is 1.5%. These results will depend on the particular extraction system which is being used.
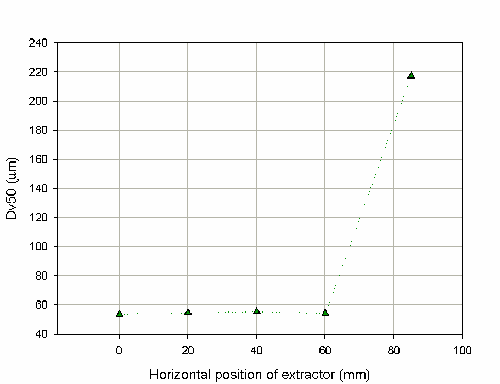
As it is sometimes difficult to position the nozzle to fire precisely into the center of the extraction hood, the effect of varying the horizontal position of the extractor was also investigated. Figure 9 shows that the Dv50 is stable until the distance exceeds 80mm from the center of the extraction hood. However, this limiting distance will be dependent on the design of the extraction system. In this case, the hood has a radius of 120mm and during the measurement at 85mm it was noticeable that liquid was dripping off the hood and falling back through the laser beam. The measurement is therefore insensitive to the horizontal position of the hood until the positioning of the hood begins to make the measurements worse due to spray impaction. This insensitivity is demonstrated by the COV on the Dv50 of 1.4% for the measurement up to the distance of 60mm.
|
|
The determination of refractive index is relatively simple for nasal sprays, as most are aqueous-based. Determining of refractive index using a refractometer is also simpler for liquids. The imaginary refractive index (absorption) of the droplets will generally be zero. The refractive index of the dispersant is also easily obtained as it is almost always air.
Assessment of the linearity of the laser diffraction technique is not required [2,3], as few particle sizing techniques exhibit a linear response to particle diameter. However, the variation of particle size with obscuration or concentration should be considered. The measured particle size should be independent of the concentration of droplets in the beam. In the case of sprays it is not usually possible to change the concentration of the spray and as such it is not always possible to assess the effect of concentration on particle size. In order to overcome problems associated with measurements at high concentrations, the Spraytec has a multiple scattering correction which allows measurement to be carried out at obscurations up to 95%. However, the FDA guidance document [1] suggests that measurements should be carried out at obscurations where multiple scattering does not occur. The lack of multiple scattering effects can be confirmed by carrying out the analysis with and without the multiple scattering correction applied. In conditions where there is no multiple scattering there will be no difference in the results.
There are slightly different definitions for reproducibility in the standards literature. For instance, NIST define reproducibility as a measure of precision of repeated measurements of the same sample where one of the following is varied; instrument, time, measurement conditions, user or location [5]. The ISO 13320 standard defines reproducibility as a measure of precision between different instruments [4].
In spray measurements it is not possible to measure the same sample more than once, and in most cases the option to measure on more than one instrument is not available. In this case the reproducibility of the device was therefore investigated by measuring six repeated actuations of the device. The COV for all six actuations was then calculated.
| Actuation number | Dv10 μm | Dv50 μm | Dv90 μm |
|---|---|---|---|
| 1 | 20.93 | 54.16 | 109.6 |
| 2 | 21.24 | 53.96 | 109.3 |
| 3 | 21.74 | 54.23 | 110.2 |
| 4 | 20.68 | 54.29 | 110.0 |
| 5 | 20.90 | 54.13 | 109.7 |
| 6 | 21.74 | 54.93 | 111.7 |
| Mean | 21.21 | 54.28 | 110.1 |
| %RSD | 1.95 | 0.57 | 0.70 |
Table 1 shows the Dv10, Dv50 and Dv90 for six repeated actuations of the same device using a medium velocity actuation profile. In this case the %RSD is 0.57% for the Dv50 and within the limits stated in ISO13320, USP<429> and EP 2.9.31.
The intermediate precision of a method is determined by carrying out a repeat of the reproducibility test using a different instrument or operator. The results of both reproducibility tests should then be combined in order to calculate a mean and RSD to cover both experiments.
Table 2 shows the second set of measurements carried out on a different day, in order to assess intermediate precision. For this set of data on its own, the relative standard deviations for the Dv10, Dv50 and Dv90 are all within the limits set out in ISO13320, USP<429> and EP 2.9.31.
| Actuation number | Dv10 | Dv50 | Dv50 |
|---|---|---|---|
| 1 | 20.45 | 52.36 | 104.0 |
| 2 | 20.59 | 52.94 | 105.0 |
| 3 | 20.30 | 52.23 | 104.1 |
| 4 | 19.86 | 51.44 | 102.9 |
| 5 | 19.87 | 51.32 | 103.1 |
| 6 | 19.77 | 50.96 | 102.4 |
| Mean | 20.14 | 51.87 | 103.6 |
| %RSD | 1.73 | 1.45 | 0.92 |
In order to assess the intermediate precision, both sets of data must be combined and a coefficient of variance calculated which covers all of the data. Table 3 shows the mean, standard deviation and %COV for the Dv10, 50 and 90 for the combined data sets. Although the variances are higher than those calculated from the individual sets of measurements, they are still within the required limits for laser diffraction measurements as stated ISO13320, USP<429> and EP 2.9.31. This variation takes into account the accumulative error caused by small operation-to-operator changes in the actuator and extraction system set-up as well as variations in the output of the pump itself.
| Dv10 | Dv50 | Dv90 | |
|---|---|---|---|
| Mean | 20.71 | 53.17 | 107.09 |
| Standard deviation | 0.67 | 1.36 | 3.48 |
| %COV | 3.21 | 2.56 | 3.25 |
This application note has investigated the sensitivity of a nasal spray measurement to sampling effects, sample preparation and the deliberate variation of various parameters. The effects of sampling can be easily controlled by setting the position and angle of the nasal spray in the Spraytec software.
The robustness of measurements to variations in horizontal position of the actuator and to the position of the extraction systems was also investigated. The measured particle size was found to be insensitive to the horizontal position of the actuator and the effective range of the extraction system was determined.
The effects of sample preparation were discussed in terms of the actuation conditions. In this case the device was insensitive to variations in the actuation conditions, although this test will depend strongly on the device measured. Finally, how to assess the reproducibility and intermediate precision was described.