One of the important aspects of nasal pump spray performance which should be considered during product development is the rate at which the dose emitted from the spray pump decreases, or tails-off, after the point where the labeled number of doses has been delivered. The US Food and Drug Administration's (FDA's) Chemistry and Manufacturing Controls (CMC) "Guidance for Industry" document for nasal spray and other spray drug products recommends the profiling of spray products near the point of container exhaustion1. Profiles for Spray Content Uniformity (SCU), droplet particle size and the drug particle size for suspension products are suggested for each individual spray dispensed after the labelled number of sprays until no more sprays are possible. These measurements can then be used to help determine if the target fill level or any proposed overfill of the nasal spray container is justified.
In this application note, the Malvern Spraytec laser diffraction system (figure 1) has been used to monitor the changes in droplet particle size during each life stage of a nasal spray pump (the beginning, middle and end stages). The results allow the reproducibility of droplet formation to be assessed during the lifetime of the device. It is also possible to assess how the device output changes during tail-off, enabling an assessment of how drug delivery may be impacted.

|
The output of a typical nasal spray pump spray product was measured from initial prime through to the point of being empty using the Malvern Spraytec system. Two separate pumps were characterized; one using a distance of 30mm between the nasal spray pump and the Spraytec measurement zone and another using a distance of 60mm. Measurements of the entire spray event were made for each actuation. From this data the average particle size delivered during the stable phase of spray delivery was calculated2. The Solo for Spraytec actuator was used to control the velocity of actuation during each measurement.
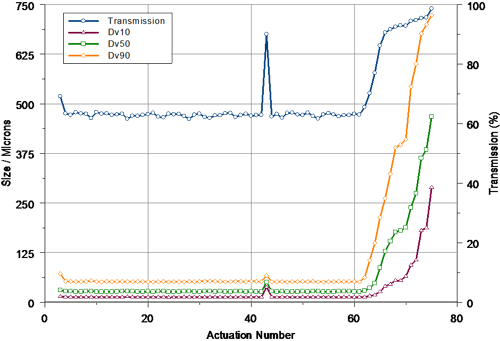
Figure 2 shows the profile obtained during the actuation of the first pump at a measurement distance of 30mm. Three priming actuations were carried out prior to acquiring the first of the Spraytec measurements. The particle size observed for the first full spray emitted from the device was slightly larger than that observed for subsequent actuations - this suggests that the pump was not fully primed. Following this, one failed actuation (actuation 43) was observed prior to the tail-off stage. For this actuation, the measured transmission was higher than expected, showing the spray concentration to be low. This suggests that there was a problem with the metering of the liquid in the pump. However, even including this actuation, the output of the device was very reproducible, with a coefficient of variation of only 1.13% being observed for the median particle size (Dv50) prior to tail-off.
|
In this case, the pump system was designed to deliver 50 doses. The tail-off profile beyond actuation 50 shows that complete atomization of the spray was observed until actuation 61. Beyond this, the concentration started to decrease (as evidenced by an increase in the measured transmission) and the particle size increased. The last measurable spray emitted by the pump related to actuation 75.
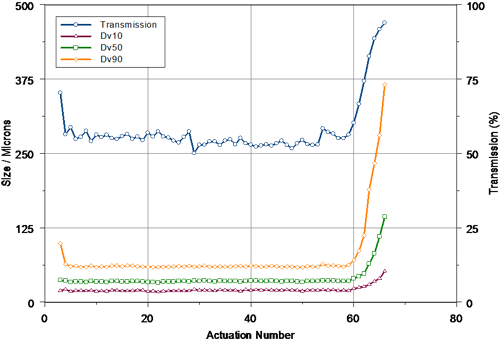
In order to determine if the results obtained above provided a realistic measure of the tail-off characteristics of the pump system, a second device was characterized, this time at a measurement distance of 60mm. The data obtained for this second set of measurements is shown in figure 3. Following the priming stage, reproducible spray delivery was observed up to actuation 59. The measured Dv50 was larger than that observed at 30mm, perhaps relating to droplet coalescence within the spray plume. Again, the output of the pump was very reproducible, with a coefficient of variation of only 1.0% being observed for the Dv50 prior to tail-off.
|
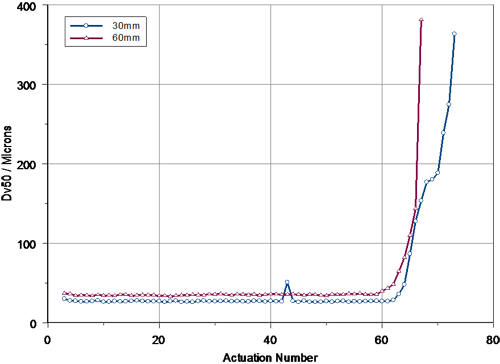
A comparison of the tail-off profiles observed at 30mm and 60mm is presented in figure 4. This confirms that the pump performance was similar for each set of measurements, although the observed tail-off was perhaps more rapid at 60mm. The increased rate of tail-off at 60mm possibly relates to changes in the spray plume geometry during the final set of actuations. This leads to a more rapid reduction in the concentration of particles observed in the Spraytec measurement zone at a distance of 60mm compared to that observed at 30mm.
|
The FDA's CMC guidance for spray drug products confirms the importance of understanding the tail-off performance of nasal pump sprays close to the point of exhaustion of the pump container. The Malvern Spraytec provides a robust, reproducible means of measuring the spray droplet size distribution observed during all of the life stages of pump operation, including during tail-off. This can aid with understanding how the bioavailability of the drug substance may be impacted by changes in pump operation both up to and beyond the label-claim number of doses.