The zeta potential of a colloidal dispersion is a measure of the electrostatic repulsion between particles and can be used as an indicator of dispersion stability.
If all the particles in suspension have a large negative or positive zeta potential then they will tend to repel each other and there is no tendency for the particles to come together. However, if the particles have low zeta potential values then there is no force to prevent the particles flocculating or aggregating.
Zeta potential is influenced by pH, ionic strength (the concentration and type of ions present) and the concentration of any charged molecules in the dispersant. The effect of the pH, or ionic strength of the medium or the concentration of an additive on the zeta potential can give information to assist with formulating the product to give maximum stability.
Further information on zeta potential can be found in the technical note "Zeta Potential: An Introduction in 30 Minutes" available on the Malvern web site.
The measurement of zeta potential can be performed on a suitable Zetasizer Nano instrument using the technique of laser Doppler electrophoresis. Further information on this technique can be found in the technical note "Measuring Zeta Potential: Laser Doppler Electrophoresis" available on the Malvern web site.
The isoelectric point (IEP) is defined as the point where the surface carries no net electrical charge. For surfaces with no specifically adsorbed ions, this will be the as the point of zero zeta potential. For a sample which is electrostatically stabilized, the IEP is often the point of least stability due to the repulsive forces being weakest. This may be important when considering the shelf life of a product, as normally the sample needs to be formulated to to have a significal positive or negative zeta potential.
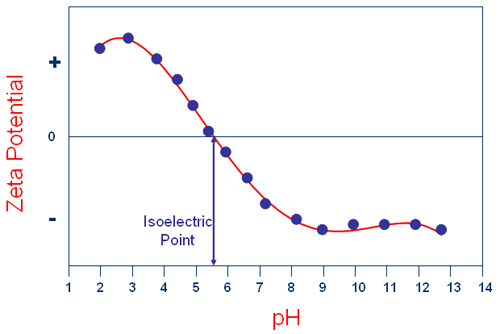
Figure 1 shows a typical plot of the zeta potential of a sample measured as a function of pH. In this example, the isoelectric point of the sample is at approximately pH 5.5. In addition, the plot can be used to predict that the sample should be stable at the extremes of pH.
|
For example, at pH values less than 4, there is significant positive charge present. In addition, at pH values greater than 8, there is significant negative charge present. At these extremes of pH, the forces resulting from this electrostatic repulsion should be sufficient for the sample to resist flocculation if the zeta potential has a value greater than + 30mV or less than -30mV.
In order to determine whether a sample is sensitive to the concentrations of components in the formulation, the conditions of the sample need to be altered and the effect on the zeta potential monitored. This procedure can be carried out manually. However, automatic determination is less time consuming and more desirable and can be achieved by combining a Zetasizer Nano instrument with a Multi Purpose Titrator MPT2.
The MPT-2 is an integrated system designed to automate changes in the composition of the sample and then transfer the sample to the optics unit for measurement of the size, zeta potential and intensity.
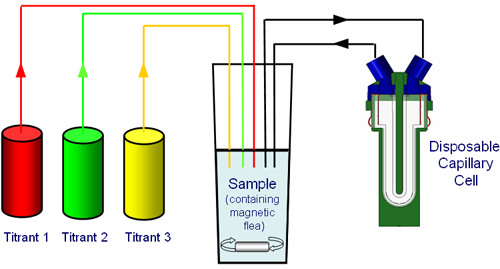
Figure 2 shows a typical titration schematic for the MPT2 linked to a Zetasizer Nano. The MPT2 incorporates a pH meter and has the ability for three titrants to be used to change the composition of the sample and can be programmed to perform the following titration types:
|
The titrants used will depend upon the type of titration requested. For example, if the effect of changing the pH on the zeta potential of a sample is to be studied, the titrants would be acid and base. If the change in conductivity wanted to be studied, an appropriate salt would be used as a titrant in a logarithmic additive titration.
After each change in sample conditions the system automatically circulates the sample to the measurement capillary cell situated in the Zetasizer Nano for the measurements to be made. The measurement of particle size, zeta potential or the intensity of scattered light can be programmed depending upon which Zetasizer Nano instrument is used.
The following examples were all measured on a Zetasizer Nano ZS instrument in conjunction with an MPT-2, with the system set at a temperature of 20°C.
| Parameter | Value |
|---|---|
| Titrant 1 | 0.25M HCl |
| Titrant 2 | 0.025M HCl |
| Titrant 3 | 0.25M NaOH |
| Start pH | 2 |
| End pH | 9 |
| pH increment | 0.5 |
| Number of measurements at each titration point | 3 |
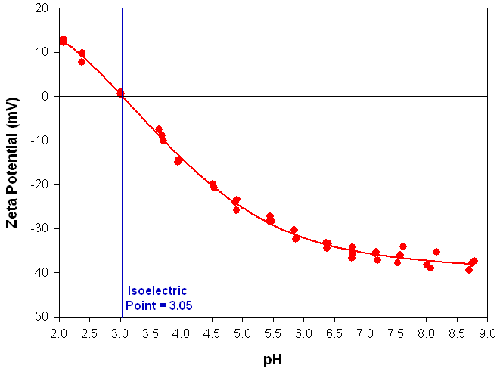
Figure 3 shows the zeta potential measured as a function of pH for a titanium dioxide sample dispersed in deionized water. The titration conditions are defined in table 1. Two concentrations of HCl titrant were used to increase the accuracy of setting the pH around neutrality. The repeatability of the measurements at each pH increment was excellent and the sample had an isoelectric point at pH 3.05.
|
|
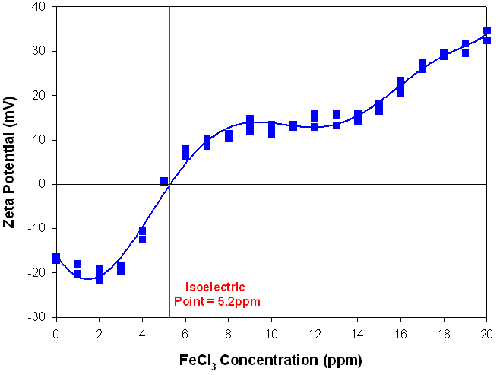
Table 2 summarizes the conditions used for a linear additive titration. A sample of magnesium hydroxide was titrated with FeCl3 to investigate the effect on dispersion conditions. A stock FeCl3 solution of 1000ppm was used as the titrant and 3 repeat measurements were performed at each titration point. Figure 4 shows that the repeatability of the results was excellent and that the magnesium hydroxide sample had an isoelectric point at 5.2ppm of FeCl3.
| Parameter | Value |
|---|---|
| Titrant 1 | 1000ppm FeCl3 |
| Start additive concentration | 0ppm |
| End additive concentration | 20 |
| Additive concentration increment | 1 |
| Number of measurements at each titration point | 3 |
The determination of the iso-electric point is an important parameter to help understand the stability of colloidal dispersions. The Multi Purpose Titrator MPT2 used in conjunction with a Zetasizer Nano instrument allows for the automatic determination of isoelectric points.