
|
Viscosity is a measure of the tendency of a liquid to resist flow, more correctly termed the coefficient of viscosity, η. (The Greek letter êta) This is the ratio of the shear stress to shear rate at a simple steady shear rate. When this term is constant at different values of shear rate, then the liquid is known as Newtonian. If the coefficient varies as a function of shear rate then the liquid is known as non-Newtonian.
The shear rate is the velocity difference between two liquid layers subjected to a shear stress, divided by the distance between the layers. For a rotational viscometer a high shear rate is when the disk is rotating quickly, when using a relatively small plate spacing.
Dynamic Light Scattering (DLS) measures size by observing the random movement of particles undergoing Brownian motion. For spherical particles that do not sediment noticeably during the measurement, this movement only depends on the size of the particles, the absolute temperature and the viscosity of the medium.
DH α 1/ η.d (1)
DH = Hydrodynamic diameter
η = viscosity coefficient
d = diffusion speed of particles undergoing Brownian motion
The main reason we need to measure and control the temperature precisely is that the viscosity is usually highly dependent on the temperature. For water at about ambient temperature, the change in viscosity is about 2.4% per degree Celsius.
Equation 1 shows that the error in the value used for viscosity is reflected in the error in the reported size. If the viscosity used for the calculation is 10% too high, the size calculated will be 10% too low.
This is not usually an issue if the sample is dilute and is dispersed in a fluid of known viscosity such as water. The addition of a salt at low concentration has a minor effect on viscosity and so can usually be ignored. However even 100mM Sodium Chloride will increase the viscosity of water by 1%, so if this is ignored the result will be reported as 1% too high.
An additive such as 3.5% Ethylene Glycol will increase the viscosity by 10%, and a buffer such as 'Tris' (Tris Hydroxymethylaminoethane) at isotonic concentration will increase the viscosity by 5%.
Solutions of simple salts will be Newtonian fluids, and as such, the value of the viscosity measured by any suitably accurate method can be used, whatever the shear rate. This value is used as the viscosity of the dispersant for the dynamic light scattering measurement.
|
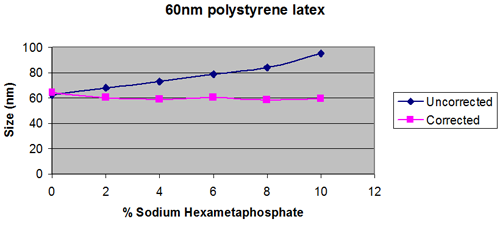
This is assuming that the dispersed particles are dilute, so that the viscosity of the bulk sample is the same as the viscosity of the dispersant on its own. (Figure 1)
|
If the dispersant contains polymers, polyelectrolytes or higher molecular weight surfactants, then it is possible that the viscosity measured will depend on the measurement technique, as each method will use a different, and often unknown shear rate.
The recommendation is to use the same measurement method for all samples to give the best comparability of size results.
At higher concentrations, the apparent size reported may be seen to depend on the sample concentration. Two mechanisms can contribute to this; multiple scattering and particle interactions.
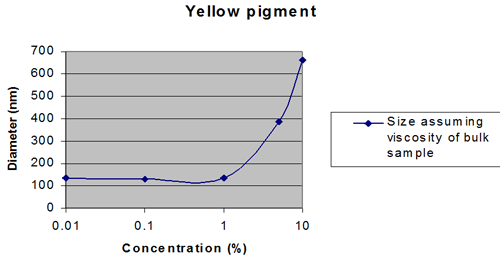
This can be the dominating effect for high refractive index samples such as pigments. It is minimized as far as possible using the NIBS optics incorporated in the Zetasizer Nano S and ZS. For samples with a refractive index close to that of the dispersant, multiple scattering may compensated for up to 40wt%, whereas for samples with a high refractive index, the size measured can be affected at concentrations below 1wt%.
As particle concentration increases, the measured viscosity can increase due to short and long-range interactions between particles.
It has been proposed that the bulk viscosity of a sample can be used, rather than the dispersant viscosity to compensate for the effects of sample concentration.
Whether the bulk viscosity can be used will depend on whether multiple scattering or particle interactions dominate. The only way to ensure that the result is independent of concentration is to do an experiment to plot apparent size, as a function of concentration.
This will show whether the viscosity of the dispersant is more appropriate, or whether using the bulk viscosity give a wider working concentration range.
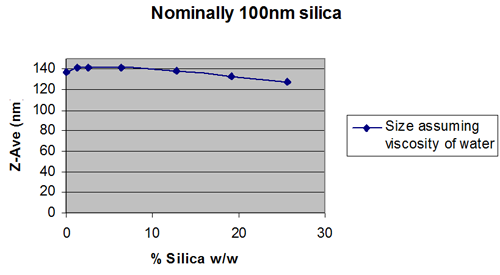
Figures 2 and 3 show extreme cases.
|
|
This method uses a disk or cylinder that is rotated either in a large volume of fluid, or the fluid is contained between the rotating component and a stationary plate or larger cylinder. In both cases, the turning force or torque required to drive the disk or cylinder at a known steady rate is measured, and this is converted to the fluid viscosity by well known relationships.
These are well accepted methods, however a number of disks or cylinders are required to cover a range of viscosities, and the error at low viscosities can be in the order of 10%, even for the best systems.
There are two types. One involves pumping fluid at a known rate through a capillary tube and measuring the pressure drop. The other uses gravity to cause fluid to pass down a capillary and the time for the fluid to drain through a measured length of capillary is timed. Both methods require calibration with a fluid of know viscosity.
These methods are simple in principle, however in practice they are inconvenient because of the very careful cleaning procedure required. In addition for the gravity capillary, the density must be known as the value measured is the kinetic rather than the dynamic viscosity. Each capillary will only cover a narrow range of viscosities, so a number of tubes are usually required.
The cup type viscometer, sometime called the Ford cup, is related to the capillary viscometer. It uses the time taken for a fluid in a cup of a specific capacity to drain from a hole in the cup to give an indication of the viscosity, rather than a true viscosity reading.
This technique is usually only used for viscous fluids, and is traditionally used in the paints or inks industry. A selection of cup types is required to cover a wide viscosity range.
This consists of a ball, needle or cylinder falling due to gravity down a column of the fluid to be measured. The time to fall a marked distance is measured manually or automatically and some more expensive versions have built in temperature control. To cover a wide viscosity range, the density of the ball or needle can be changed, or the capillary can be tilted so the ball runs more slowly down the inclined plane. The later case is mostly suitable for solutions, as particles in the fluid interfere with the free run of the ball along the capillary wall
There are two basic types
The resonant frequency of a probe vibrating at a relatively high frequency will change when immersed in a fluid. The frequency change will depend on the viscosity of the fluid. This method is simple to use, but the range of viscosities measured is low, 4mPa.s to a few 10's of mPa.s, and the accuracy also low at about +/- 10%.
The SV-10 uses a frequency of 30Hz, which are maintained at a constant frequency and amplitude. There is a damping effect related to the viscosity of the fluid, which reduces the amplitude, and the power required to keep the sensors vibrating at the original amplitude is measured and converted into the viscosity.
The SV-10 has two gold plated paddle type sensors 12mm in diameter that are immersed into the sample to be measured.
These paddles are in a tuning fork arrangement so when stimulated by an electromagnetic drive the paddles vibrate at a constant frequency. The amplitude of vibration is detected and sufficient current is applied to the electromagnetic drive to maintain a constant amplitude. The sample viscosity causes the vibration to be damped and the current required to maintain the vibration amplitude is measured continuously, and converted into a viscosity on the display.
The system takes 15 seconds to stabilize, after which a continuous reading of viscosity is displayed.
The value actually measured is dependent on the sample density. To get a value comparable with the dynamic viscosity measured for example by a disk and plate rotational viscometer, the value displayed must be divided by the density.
There a number of ways of measuring density. Weighing a measured volume from a micropipette, say 5mL, is suitable for low viscosity solutions such as aqueous buffers, and with care accuracies of about 1% can be obtained. Some balances have density accessories and these can be very accurate, but are a little time consuming to set up.
One of the most convenient methods is the hand held density meter. These are fast and accurate, but more expensive than the other methods. Examples are the DA-130N manufactured by Kyoto electronics. This model is also distributed by Mettler Toledo as the Densito 30PX.
VIS5001
SV-10 Fast, continuous measurement of the viscosity of fluids from 0.3 to 10,000mPa.s. Minimum sample volume 10mL, measurement repeatability 1%
Includes: Viscometer with display unit, temperature probe and 10 x 35mL polycarbonate sample cups, 10 x 10mL polycarbonate sample cups, 2 x 13mL glass sample cups, Thermostatted water jacket (Water bath required but not included), measurement arm stop, Data communication software for PC with RS232C cable, manual, universal 12v mains power adaptor.
|
This requires connection to a water bath that can supply recirculating water to the jacket. This accessory is only compatible with the 10mL polycarbonate sample cell and the 13mL glass sample cell.
This cell can be used with a protection device for the vibrating paddles, to avoid accidental damage, however these cells and the protection device are not compatible with the thermostatted water jacket.
The lowest volume sample cup, for use with aqueous samples.
Compatible with all sample types.
The software enables a continuous display of the viscosity and the temperature from the measurement unit to be displayed on a PC. This requires a free RS232 9 pin socket on the PC.
If a temperature ramp is applied to the sample, this enables a complete profile of viscosity as a function of temperature and time to be displayed.
See Rheology price list for a range of viscosity standards.
Distilled or demineralized water can be used as a calibration standard for samples between 0.3 and 100mPa.s