This Technical Note offers a brief technical overview of MicroCal iTC200 system and applications.
MicroCal iTC200 isothermal titration calorimeter (ITC) (Fig 1) allows direct and label-free quantification of binding affinity and thermodynamics. Heat released or absorbed during biochemical binding events is measured directly, giving information about relative binding affinity (KD), stoichiometry (n), enthalpy (ΔH), and entropy (ΔS) in a single experiment. This data reveal the forces that drive complex formation, providing deeper insights into structure-function relationships and the mechanisms of binding.
Based on a solution-based technology requiring no assay development, MicroCal iTC200 provides results quickly and is an essential tool for any research laboratory studying biomolecular interactions. The exceptional sensitivity and data quality gives confidence in results.
|
With MicroCal iTC200, syringe and cell cleaning functions are semi- automated and controlled requiring minimal operator involvement. MicroCal iTC200 equlibrates quickly between sample runs and cleaning cycles permitting 10 experiments/day with typical samples and protocols. The sample and reference cells are made from Hastelloy™ alloy, a nonreactive material chosen for its excellent chemical resistance and applicability with biological samples. Three different user-selectable titration response times (feedback modes) provides application versatility and variable mixing speed permits the use of a variety of sample types.
ITC measures affinity, stoichiometry, enthalpy and entropy of the interaction of binding partners in their native state without requiring modification of the components with fluorescent tags or requiring immobilization. Other than preparing samples and reagents to appropriate concentrations, there is no assay development needed and the time to first result is fast.
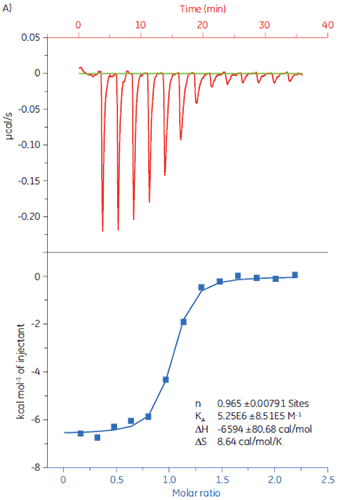
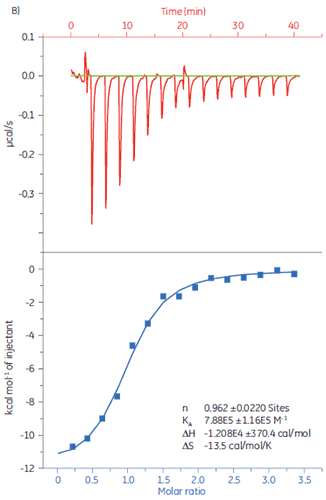
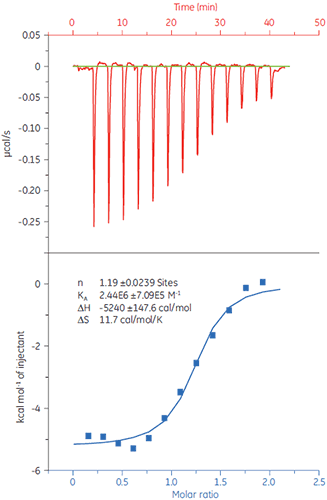
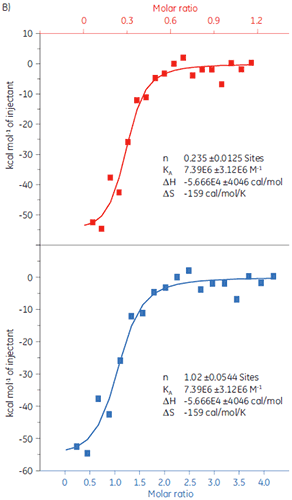
To demonstrate how ITC can be used to measure all binding parameters and provide insight into the mechanism of binding, the interaction of peptides with the protein target Bcl-2 (name derived from B-cell lymphoma 2) has been studied with MicroCal iTC200.
|
|
Experiments were carried out at 25 °C. The sample cell was filled with Bcl-2 (30 µM solution) in 50 mM HEPES, pH 7.4, 100 mM NaCl, 0.5mM TCEP, and 5% DMSO. The peptides were diluted to a concentration of 250 µM in the same buffer. The injection volumes were 3 µl each, injection time 6 s, and a 150 s delay between each injection. The results are shown in Figure 2.
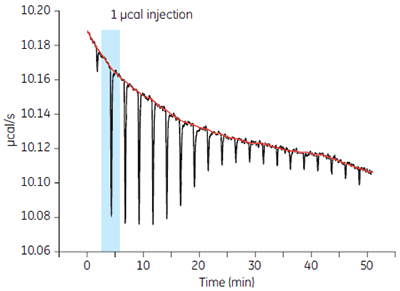
The sensitivity of MicroCal iTC200 provides excellent signal-to-noise to generate high quality data even at the low protein concentrations required for high affinity interactions or so called “tight binders.”
When studying tight binders, the experimental challenge is using the minimum protein concentration that will produce a heat change that can be measure with good signal-to-noise. A 1 µcal injection with excellent signal-to-noise is shown in Figure 3. There is a relatively low concentration of protein, 3 µM carbonic anhydrase in the sample cell where the typical recommended protein concentration is 10 µM. Even at a protein concentration of 3 µM, a strong signal is generated with MicroCal iTC200.
|
The ability to generate high quality data of small volumes of low protein concentration samples conserves precious sample. MicroCal iTC200 requires 280 µL of sample and heats can be measured for protein concentrations as low as 1.25 µM (Fig 4).
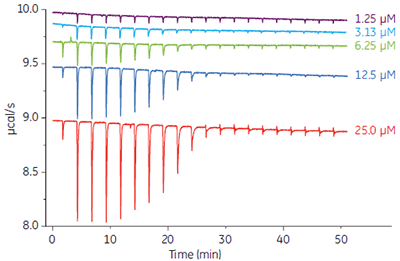
|
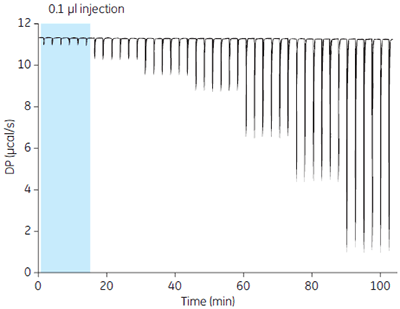
The pipette assembly of MicroCal iTC200 delivers precise volumes of titrant to the reaction volume in the sample cell (Fig 5). MicroCal iTC200 delivers < 1% variability at an injection volume of 1.5 µl and is even capable of 0.1 µl injection volume with good precision.
When performing ITC experiments where the data from a series of injections are used to generate a result, good injection volume precision reduces scatter in the final calculated results.
|
The excellent sensitivity of MicroCal iTC200 permits experimentation over a wide range of affinities from weak to tight binders. For example, the binding thermodynamics for 11 inhibitors to human CA2 was studied using MicroCal iTC200. The raw data as well as integrated heats from the interaction of human CA2 and 4-carboxybenzenesulfonamide are shown in Figure 6. The calculated values for affinity and enthalpy for the 11 inhibitors are in Table 1. The affinities for the 11 substances binding to human CA2 span a 5000-fold difference in affinities.
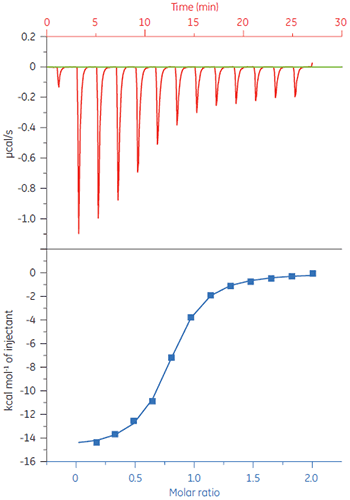
|
| No. | Compound |
KD (µM) |
∆H
(kcal mol-1) |
[Protein]
(µM) |
[Compound]
(µM) |
|---|---|---|---|---|---|
| 1 | Ethoxzolamide | 0.00083 | - 14.4 | 10 | 107 |
| 2 | Acetozolamide | 0.013 | - 14.7 | 10 | 97 |
| 3 | Methazolamide | 0.023 | - 13.0 | 10 | 115 |
| 4 | 4-nitrobenzenesulfonamide | 0.093 | - 13.8 | 10 | 100 |
| 5 | p-toluenesulfonamide | 0.58 | - 10.0 | 29 | 282 |
| 6 | Azosulfamide | 0.49 | - 17.6 | 29 | 312 |
| 7 | Benzenesulfonamide | 1.4 | - 10.8 | 29 | 309 |
| 8 | 4-carboxybenzenesulfonamide | 0.84 | - 15.0 | 29 | 297 |
| 9 | 2-aminobenzenesulfonamide | 1.9 | - 12.0 | 29 | 273 |
| 10 | Furosemide | 1.3 | - 4.2 | 30 | 255 |
| 11 | Sulfanilamide | 4.0 | - 10.1 | 29 | 405 |
MicroCal iTC200 instrument control and data analysis software enhances data quality and streamlines data analysis. The control software incorporates all the tools you need to go from experimental design to final results quickly and easily. The data analysis software has a robust baseline fitting algorithm eliminating the need for tedious manual manipulation post-run. Experimental controls are automatically subtracted saving time.
|
MicroCal iTC200 can be upgraded to MicroCal Auto-iTC200 proving complete automation of the ITC experiment from sample loading to data analysis. Walk-away operation frees up the scientist for other more manual tasks. With a system that is upgradeable to full automation, the needs of a growing laboratory can be met.
ITC is used in a variety of applications for studying the interactions of various binding partners from simply assessing the activity of a target protein to revealing the forces that drive complex formation to describe how the interaction occurs at the molecular level. The information can be used together with structural data to understand structure-function relationships. The types of interactions that can be studied are not limited to proteins and there are numerous references in the literature where ITC has been used to understand how nucleic acids and lipids, as well as proteins, function in biological systems.
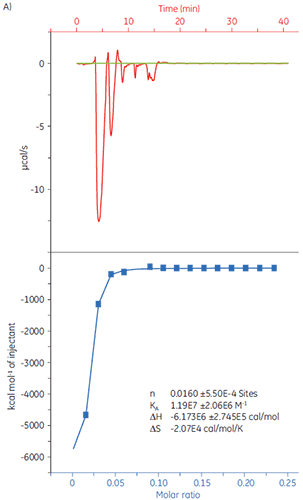
It is important to rule out false positives from a primary screening at an early stage. ITC can be used to verify hits and exclude any false negatives, saving considerable time and effort later in the process. As an example 20 µM solution of target protein was loaded into the sample cell of MicroCal iTC200 and titrated with Compound X (Fig 8). The KD was determined to 4.9 µM, which correlated well with studies performed with Biacore™ systems and NMR, thus confirming that Compound X is suitable for further studies.
|
When the same target solution was titrated with Compound Y, the results were very different (Fig 9). In the left panel, Compound Y was titrated with target protein. The isotherm shows a binding affinity of 120 nM, but the binding enthalpy was about 1000-fold larger than expected and the stoichiometry value (0.01) very low. In the right panel, the same drug candidate was titrated with bovine serum albumin (BSA). Taken together, the results indicate nonspecific activity and this compound is not suitable for further development. Based on these experiments, Compound Y was considered to be a false positive and was removed for further study.
|
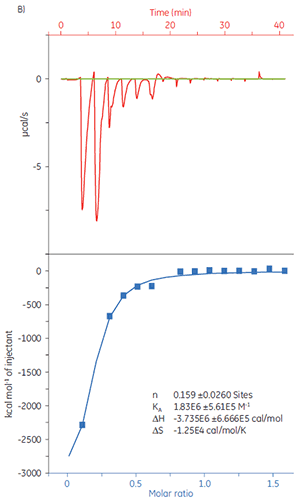
|
ITC can be used to assess the activity level of a target protein before use in high throughput screening. Two batches of a target protein were compared using MicroCal iTC200 by titration with a standard peptide (Fig 10).
Fig 10. Two batches of target protein (A and B, respectively) titrated with a standard peptide. The sample cell contained the protein at a 10 µM concentration, and the peptide solution was 50 µM.
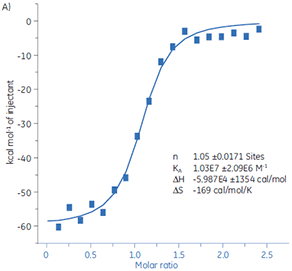
|
|
The result for Batch 1 (Fig 10A) demonstrates a typical isotherm with a KD of 97 nM and n = 1. The second batch (Fig 10B) has a KD of 135 nM but n is only 0.23. The analysis of the same set of data but with an estimated protein concentration of 2.3 µM gives the same KD value and n = 1. This indicates that 75% of the Batch 2 protein was inactive. The Batch 2 protein was rejected for use in the screening.
The data shown in Figure 2 were kindly provided by Dr. Lin Gao, Hoffman-La Roche, Nutley, NJ, USA.
| Sample volume | 280 μL |
| Equilibration time from 25°C to 5°C | < 6 min |
| Response time | 10 s* |
| Throughput | 8–12 per 8 h day |
| Injection volume precision | < 1% at 2 μL |
| Injection syringe volume | 40 μL |
| Cell material | Hastelloy |
| Cell configuration | Coin-shaped |
| Cell volume | 200 μL |
| Noise | 0.2 ncal/s† |
| Temperature range | 2°C to 80°C |
| Temperature stability at 25°C | ± 0.00015°C |
| Multiple feedback modes | Yes (passive, high gain, low gain) |
| Automated upgrade available | Yes |
| Dimensions, calorimeter
(W × H × D) |
21.0 × 33.7 × 34.9 cm |
| Weight | 9.4 kg |
* High feedback
† Cell temperature = 25°C, no feedback, 5 s filter period, stir speed = 1000 rpm