Spidroins are a unique family of large, structural proteins that make up the bulk of spider silk fibers. The mechanical strength and elasticity of spider silk fibers have led to their successful use in the regeneration of peripheral nerves in rats [1]. Recombinant spider silk proteins have also shown potential for use in drug delivery systems [2]. Despite the wide range of medical applications that spider silk proteins offer, the production of these proteins on an industrial scale has only become possible fairly recently.
The evolution of spidroins has been influenced strongly by a requirement for high tensile strength, a quality that does not necessarily guarantee conformational or chemical stability when other stresses are applied. This application note describes the use of a Zetasizer Nano ZSP to characterize the charge state of spidroins in different formulations. The earliest stages of spidroin aggregation in different formulations are then assessed using low-temperature dynamic light scattering (DLS) thermal trend experiments.

|
The zeta potential of spidroin samples was determined on a Zetasizer Nano ZSP using folded capillary cells. All electrophoretic light scattering (ELS) measurements were acquired using a fixed voltage of 75 V, or, given that the distance between the electrodes of a folded capillary cell is 6 cm, an electric field strength of 12.5 V/cm. A monomodal analysis model was applied, fast field reversal analysis being carried out alone on the samples in order to minimize the effect of joule heating. All ELS measurements were acquired at 25 °C after allowing for thermal equilibration of samples.
Samples were heated from 4 °C to 21 °C, with measurements acquired at temperature intervals of 1 °C. The system was purged with dry air during thermal trend experiments in order to minimize condensation on the cuvette wall.
Table 1 shows the results of the cumulants analysis on two different spidroin protein samples. The data shown in table 1 indicates that the presence of glycine increases the zeta potential of both spidroins. The presence of sucrose, on the other hand, lowers the zeta potential of spidroin 1 and increases that of spidroin 3.
There is a large increase in the negativity of zeta potential measured during storage of both spidroins in glycine, suggesting large changes in the global charge arrangement of the proteins as a consequence of denaturation.
| Spidroin Construct | Formulation | Initial ZP (mV) | ZP after storage (mV) |
|---|---|---|---|
| Spidroin 1 | None (1) | -5.50 | -4.17 |
| 100 mM glycine (2) | -9.87 | -15.9 | |
| 20 % sucrose (3) | -2.06 | -1.36 | |
| Spidroin 3 | None (1) | -2.36 | -4.68 |
| 100 mM glycine (2) | -6.48 | -17.9 | |
| 20 % sucrose(3) | -8.68 | -11.3 |
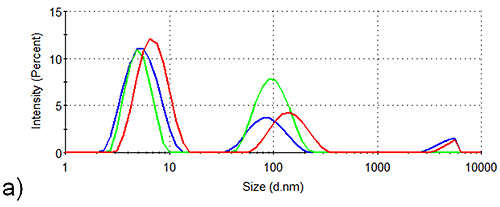
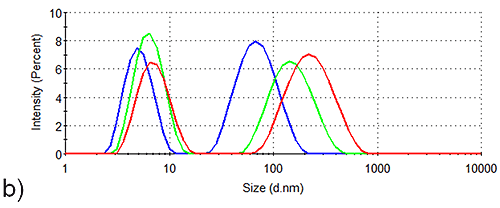
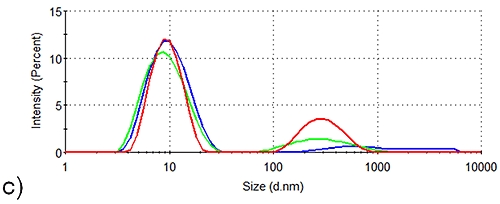
In order to assess the solution stability of the spidroin 1 formulations, thermal trend experiments were carried out. Data from these experiments are displayed in Figure 1, and indicate that the presence of sucrose increases the propensity of spidroin 1 to form large aggregates as the temperature is increased from 4 °C - 21 °C. The presence of glycine, on the other hand, clearly inhibits the formation of larger aggregates, the glycine formulation containing very few large aggregates at 4 °C, though there is some aggregate growth as the temperature is raised.
The aggregation-inhibition effect of glycine is consistent with the high zeta potential that this additive imparts on the spidroin, whilst the destabilizing effect expected fom the presence of sucrose is consistent with the lower zeta potential of the protein measured in the sucrose formulation.
Measurement of zeta potential using the Zetasizer Nano ZSP allows prediction of the aggregation propensity of protein in solution. Peltier temperature control allows DLS-assessment to be carried out at different temperatures, allowing the temperature of aggregation onset to be calculated, giving a direct measure of stability. Whilst most protein aggregation studies are carried out over a higher and wider temperature range than that used here (Zetasizers allow accurate temperature control from
0 °C - 90 °C), the high sensitivity of DLS to larger particles allows the earliest stages of aggregation to be characterized at relatively low temperatures.
[1] Allmeling, C., Jokuszies, A., Reimers, K., Kall, S., Choi, C. Y., Brandes, G., Kasper, C., Scheper, T., Guggenheim, M. & Vogt, P. M. (2008) Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Proliferation, 41, 408-420
[2] Hofer, M., Winter, G. & Myschik, J. (2012) Recombinant spider silk particles for controlled delivery of protein drugs. Biomaterials. 33, 1554-1562
|
|
|