In this application note, we present data from two typical pharmaceutical formulations. X-ray diffraction plays a vital role in measuring and hence ensuring that pharmaceutical compounds do not contain unwanted impurities, that they have the right concentrations of active ingredients and the crystalline to amorphous ratio in a mixture contributes to the desired dissolution properties. The high-quality transmission capability of Aeris, adds to the other existing features, such as ease of use, automatability, suitability for regulated environments to provide a powerful and high quality X-ray diffractometer in any laboratory setting.
![[PN13596_DSC_2023_02_14_16_23_16-1.jpg] PN13596_DSC_2023_02_14_16_23_16-1.jpg](https://dam.malvernpanalytical.com/e6ba01bd-fd70-4ce4-8ef1-b07c00f31330/PN13596_DSC_2023_02_14_16_23_16-1_Original%20file.jpg)
Figure 1: Enjoy a truly versatile powder diffraction capability in a compact state-of-the-art instrument
Transmission measurements let you minimize preferred orientation effects. They are an especially great match for low-absorbing organic materials such as those used in the pharmaceutical industry.
Aeris can be a dedicated transmission diffractometer for routine measurements or can be a versatile powder diffractometer supporting occasional transmission measurements. The high-performance de-coupled goniometer scanning technology provides highly reproducible 2θ scans suitable for transmission X-ray diffraction. Optical components can be quickly and accurately exchanged thanks to our patented PreFIX fast exchange system. Transmission through sample held between foils or in capillaries is reproducibly achieved with our precision-made sample holders.

Figure 2: Samples waiting in the Aeris loading area.
Tetracycline is in a class of medications called tetracycline antibiotics. It works by preventing the growth and spread of bacteria. The molecule C22H24N2O8 · HCl crystallizes to form a highly soluble yellow powder. For this application note, it serves as a typical example of an organic sample that is well suited to a transmission measurement.
Organic materials are highly penetrated by X-rays. For a reflection geometry, that causes depth variation of the X-ray beam into a sample, leading to peak broadening in the data.
By using a transmission geometry, the beam passes completely through the sample and the entire sample contributes to the peak intensity whilst at the same time avoiding peak broadening effects.
There are many diffraction peaks from these kinds of organic materials and narrow peak widths (high resolution) are essential for accurate material identification or derivation of other structural properties.
Figure 3.1 shows the raw data from both a transmission scan through foils containing sample (red line) and through empty foils (black line). These are subtracted to give the sample data.
Figure 3.2 shows the case where a Tetracycline hydrochloride sample is mixed with a trace of Aspirin® and this is quantified using HighScore Plus Rietveld refinement to reveal an 0.8% Aspirin® concentration.
These results show both the benefit of using the transmission mode and the sensitivity of Aeris to low concentration additives and impurities.
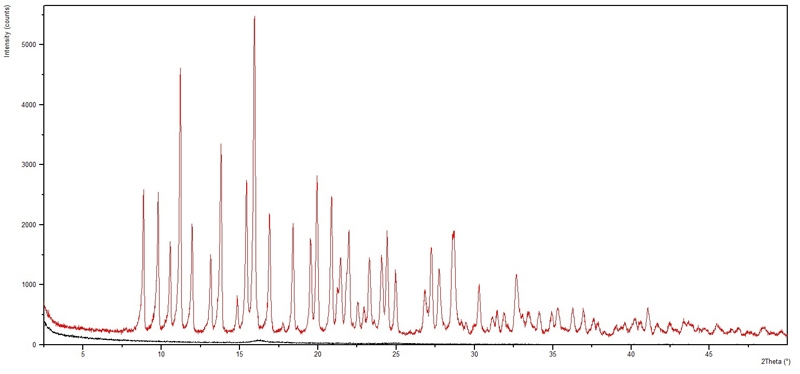
Figure 3.1: Transmission XRD measurements of tetracycline-HCL powder prepared between mylar foils showing a very good data quality
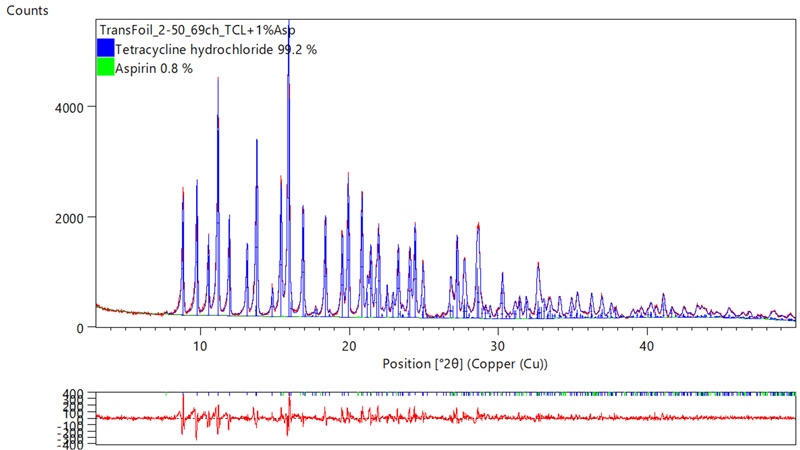
Figure 3.2: The data quality enables accurate phase analysis with good Rietveld fitting as shown on this example exported from HighScore Plus
Acetylsalicylic acid (Aspirin®) is well known as a non-steroidal anti-inflammatory drug, and in the prevention of disease associated with blood and the circulatory system. Acetylsalicylic acid is synthesized in a white crystalline powder form.
One of the common attributes of acetylsalicylic acid and many similar substances, is that the powder grains tend to be non-spherical as a result of their crystal structure and crystallization processes. Their lack of sphericalness means that they tend to settle with a preferred crystallographic orientation when pressed into tablets or deposited as powder on a holder for X-ray diffraction experiments using the reflection geometry. The relative intensities of diffraction peaks are defined by crystal structure and they could be simulated from the crystal structure. Preferred orientation seriously affects relative intensities.
For the most precise results for crystal structure analysis and phase quantification, the data should be obtained from a sample in which the crystallites are truly randomly oriented. It is easier to obtain a more random orientation distribution by measuring in transmission configuration, especially if the sample is loaded into a capillary.
The extreme preferred orientation of the Bragg-Brentano (reflection) measurement becomes clear (See figure 4.1) when comparing the data to simulated data from the structure of acetylsalicylic acid. Whilst the pattern of peak positions match in both reflection (red line) and transmission (green line) data, establishing that acetylsalicylic acid is present in both cases, the relative peak intensities are different and this arises because of a preferred orientation in the reflection sample. The comparison of both scans with a simulated scan for acetylsalicylic acid (blue) shows a significantly better match in the transmission data. The * marks indicate, for several peaks, where the measured intensities should have been in the absence of preferred orientation.
The transmission data were then used in a Rietveld refinement using HighScore Plus analysis software (see Figure 4.2). Although some residual preferred orientation effects are still evident, the data show an improved fit to the expected structure for acetylsalicylic acid.
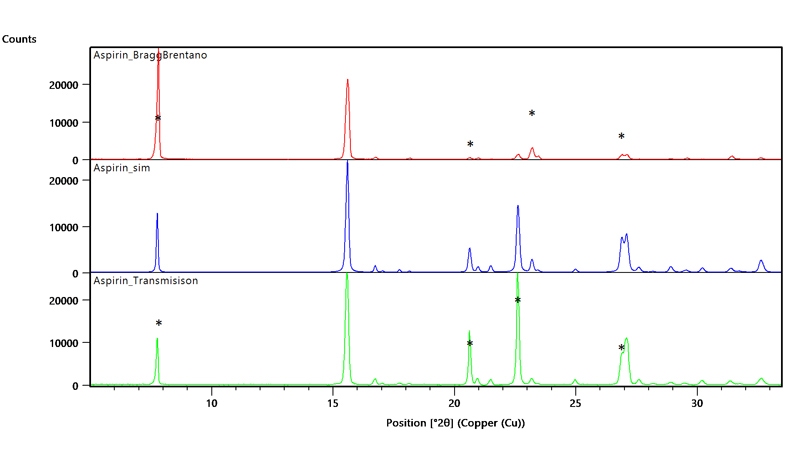
Figure 4.1: The comparison of reflection (red) and transmission(green) measurements with the simulated scan (blue). * mark indicates where the intensity should be in the absence of preferred orientation.
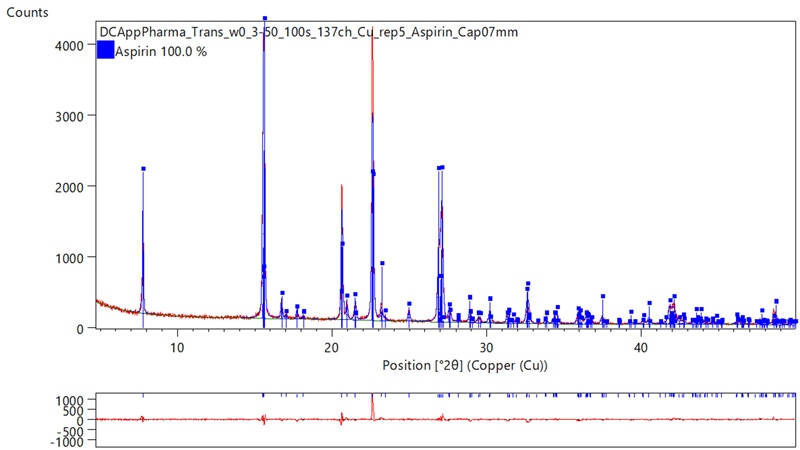
Figure 4.2: Transmission measurement of Aspirin® (acetylsalicylic acid) in 0.7 mm Kapton capillary. The data allows phase analysis including fitting and structure refinement as shown on this example exported from HighScore Plus
With Aeris you have all the capability of powder diffraction in one compact instrument. Here we show an example of transmission X-ray diffraction which demonstrates the sensitivity and versatility of Aeris and the unique quality of the data.
With our patented and unique PreFIX optical mounting system and our precision sample holders, Aeris is easily reconfigured by the user for quick comparison of data from reflection and transmission modes.
High quality data and excellent analysis results are obtained from samples mounted between foils and in capillaries. By combatting the effects of preferred orientation and low absorption, the transmission capability gives you all you need to take your crystal structure and multiphase analysis the next level.