The particle size distribution of the materials used within battery electrodes is important in defining battery power and capacity. This application note describes the use of laser diffraction measuring particle size during battery manufacture.
Login or create an account to read this application note in full.
The market for batteries is rapidly growing. The increased demand for portable electronic devices, including mobile phones and laptops, has required great advances in battery technology in order to provide a light weight, long lasting and stable power source. Battery technology is also being pushed further in electric vehicle applications, which require even more lightweight, high power and fast charging batteries.
Both primary (disposable) and secondary (rechargeable) batteries use a wide range of materials in order to achieve the required energy density at a reasonable cost. Within this, an important aspect in the design of the battery is the particle size of the materials used within the electrodes, as this helps define the battery power and capacity. In this application note, we investigated some of the common materials used in battery production, and how the particle size of these can be characterized using the technique of laser diffraction.

The performance of a battery can be characterized according to the amount of energy that it can store or the amount of power that it can produce. For a particular cell chemistry and battery size, the performance can be optimized for high energy capacity or high power [2]. The power of a battery or its current handling capacity is dependent on the rate of the reaction between the electrodes and electrolyte. This is affected by the particle size distribution of the electrode material, as this defines the available surface area. The maximum battery power can be increased by decreasing the particle size of the electrode material and increasing the surface area.
However, the energy storage capacity of a battery is dependent on the volume of electrolyte contained within the cell. As such, there has to be a balance between the space occupied by the electrodes and electrolyte. Reducing the particle size of the electrode material will increase the surface area; however it will also affect the size of the voids between the electrode particles, causing a reduction the overall electrolyte volume and the battery capacity. If these voids become too small then the contact between the electrode surfaces and the electrolyte is also reduced. This may reduce the ionic mobility within the battery, affecting the rate of reaction and reducing the battery's power. As a result, a mixture of coarse and fine particles is often used in the electrodes in order to increase surface area, through the introduction of fines, whilst also controlling the overall packing fraction of the electrode material. This allows good contact between the electrode and the electrolyte [4,5].
The particle size and distribution will also be important during production since it will affect the tap density and compressibility. These are both important parameters in the production of alkaline batteries where the cathode is compressed into shape. The detection of agglomerates will be important, as these may affect the quality of the cathode surface.
Particle size information was obtained by laser diffraction. The Mastersizer is a laser diffraction instrument with a dynamic range of 0.01μm to 3500μm. Particles, which are suspended in either liquid or air, pass through the laser beam and scatter light which is collected by detectors placed over a range of angles. Particle size information can be obtained by this method on the principle that large particles scatter light at low angles and small particles scatter light at high angles. An appropriate scattering model can be used to obtain a particle size distribution from this angular scattering data.
The wide size range of the Mastersizer is particularly useful in the measurement of battery materials, as it enables the mixtures of nanometer and micron sized particles often used in electrodes to be characterized in a single measurement. Secondly, as laser diffraction is sensitive to the volume of particles, it will be particularly sensitive to small quantities of agglomerates which may cause problems during production.
Two components of Lithium ion batteries have been characterized using the Mastersizer: lithium cobalt oxide and lithium ion phosphate. Lithium cobalt oxide is an industry standard which provides long cycle life and high energy density. However, there are environmental disadvantages to this material due to the toxicity of cobalt. Such environmental concerns are not an issue with lithium iron phosphate which has the additional advantages of low cost, greater safety and fast charging speeds. The disadvantage of lithium iron phosphate is a lower energy density, compared to Lithium cobalt oxide.
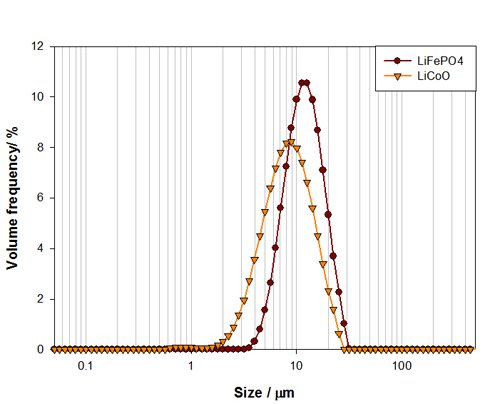
The particle size distributions for both lithium cobalt oxide and lithium iron phosphate are shown in Figure 1. From these, the median particle size and overall distribution width can be easily assessed. The Dv50 for the lithium cobalt oxide sample is 7.7μm, compared to 11.1μm for the lithium iron phosphate sample. The differences in the particle size distributions for these materials could relate to required energy density and cell size for each particular application.
|
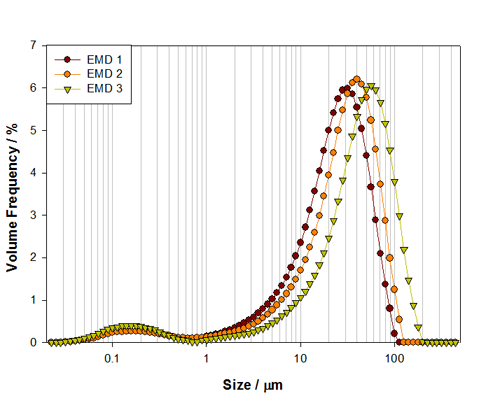
The alkaline battery material investigated in this work is a cathode material: Electrolytic Manganese Dioxide (EMD). Three grades of EMD have been measured, Figure 2, and each shows a mixture of coarse material, approximately 30μm in size, and fine particles, at around 150nm in size. Such mixtures are used to improve the performance of the battery by increasing surface area of the electrodes. The three grades show an increase in size of the main mode of the distribution, which occurs at 29μm, 37.4μm and 53.13μm for samples EMD 1, 2 and 3 respectively. As the main mode increases the surface area will decrease, but this will be counterbalanced by the presence of the fine particles, which contribute more to the surface area and therefore help to maintain the power output.
|
The size of the main mode may relate to the battery size in which the material is used. Larger battery sizes can contain electrode materials with a larger particle size, as the space into which the electrode has to fit is not so restricted. As such, a large overall surface area can be achieved without processing the cathode material to a finer particle size. This offers other possible advantages as well, in terms of electrolyte volume and ionic mobility. EMD 1 might, therefore, be used in a size AA or AAA battery, where as EMD 2 and 3 might be used in larger C or D size batteries to aid with increased capacity [6].
The particle size distributions of the electrode materials in batteries are critical in determining the performance of a battery. For a given chemistry and cell size the particle size distribution of the electrode material can be tuned to optimize the battery energy and power. To achieve high energy storage the volume of the electrolyte that must be maximized. However, when it comes to delivering high power then the surface area of the electrode is more important. It is therefore important for a battery manufacturer to be able to quickly and easily measure the particle size distribution of the electrode materials. Laser diffraction is ideally suited to characterizing these materials as the wide size range allows mixtures of nanometer and micron size materials to be characterized in a single measurement. The sensitivity to volume will also make laser diffraction an effective way of detecting any oversized or agglomerated material which could cause problems during production.
Click here to find out more about our wide range of physical, chemical, and structural solutions for battery-based energy storage and its analysis.
[1] Battery materials for ultrafast charging and discharging, B Kang, G Ceder, Nature, 12 March 2009, vol 458.
[2] Cell construction, www.mpoweruk.com
[3] The Composite Effect of Nanometer MnO2 Mixed with the Electrolytic MnO2
##MRK1412 icon 1
Yan Chao, Liu Zhuoqin, Journal of China University of GeosciencesVol 18, Issue 2, 2007,
[4] Particle-size effect of carbon powders on the discharge capacity of lithium ion batteries, Sato Y., Nakano T., Journal of Power Sources 1998, vol 75
[5] Particle size effects on temperature-dependent performance of LiCoO2 in lithium batteries, Sun Hee Choi, Ji-Won Son, Journal of Power Sources, vol 158(2) 2006
[6] www.timcal.com