Dynamic Light Scattering is an ideal method for studying micelles as it is a non-invasive technique, and can be used to study size, CMC and aggregation number. NIBS (non-invasive back scatter) technology enables characterization with little or no sample preparation combining high sensitivity with expanded concentration range.
Examples of surfactant micelle sizes (diameters) are presented: Triton X-100 at 7.5nm, Tween-20 at 8.5nm, Tween-80 at 11.7nm, Nonidet P40 at 15.4nm. The polydispersity depends on surfactant chemistry, and critical micelle concentration cmc is also specific to pH, buffer, temperature and even surfactant concentration itself. Dynamic light scattering is an ideal technique for the characterization of surfactant micelles. The results reported here illustrate the application of DLS in the study of various aspects of micelle characterization.
Surfactants belong to a class of molecules with surface active properties. This behavior is due to their amphiphilic structure which contains both a polar or hydrophilic head and a non-polar or hydrophobic tail [1].
Surfactants are normally classified according to the head group type [2]:
Adsorption of ionic surfactants onto a surface generates charge. Cationic surfactants will lead to a positively charged surface and, anionic surfactants will give a negatively charged surface.
Non-ionic surfactant molecules have no charge in aqueous media but normally consist of a highly polar region such as polyoxyethylene groups. Amphoteric surfactants develop a negative or positive charge depending on the pH of the solution.
At low concentrations, surfactant molecules are unassociated monomers. As the concentration of surfactant is increased, the attractive and repulsive forces between the molecules cause self-aggregation to occur resulting in the formation of monolayers or micelles (Figure 1). The concentration at which these micelles form is called the critical micelle concentration (cmc). The characteristics of micelles can be controlled by small changes in the chemical structure of the surfactant molecules or by varying the conditions of the disperse phase. Changes in the pH, ionic strength and temperature are all known to influence the size and shape of surfactant micelles. For some cases, the micelle size can be affected by the concentration of surfactant.
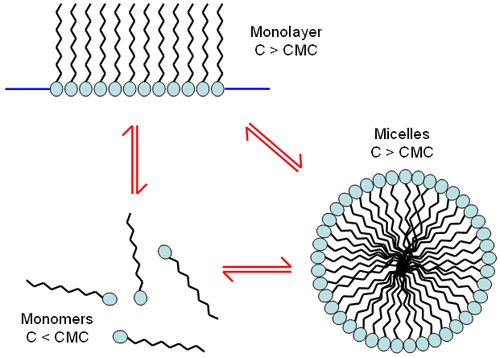
|
Surfactant micelles have been studied with various techniques [3-5]. This application note discusses the use of light scattering techniques in various aspects of surfactant characterization.
Dynamic light scattering (DLS) is a technique used for particle sizing of samples, typically in the sub-micron range. The technique measures the time-dependent fluctuations in the intensity of scattered light from a suspension of particles undergoing random, Brownian motion. Analysis of these intensity fluctuations allows for the determination of the diffusion coefficients, which in turn yield the particle size through the Stokes- Einstein equation.
Conventional DLS instruments use a detection angle of 90° and this optical configuration may not be sensitive enough for the successful measurement of surfactant micelles.
The Zetasizer Nano range of instruments incorporates non-invasive back scatter (NIBS) optics [6-8]. The scattered light is detected at an angle of 173° and this novel optics arrangement maximizes the detection of scattered light while maintaining signal quality. This provides exceptional sensitivity that is required for measuring the size of nanoparticles, such as surfactant micelles, at low concentrations.
All measurements reported in this application note were performed on a Zetasizer Nano S at 25°C. The Nano S contains a 4mW He-Ne laser operating at a wavelength of 633nm and an avalanche photodiode (APD) detector.
Particle size measurements were made of various surfactant micelles on a Zetasizer Nano S. Table 1 summarizes the z-average diameters in nanometers and the polydispersity index values for each sample. The micelles were prepared at twice the critical micelle concentration of the surfactant. The z-average diameter is the mean hydrodynamic diameter and the polydispersity index is an estimate of the width of the distribution. Both of these parameters are calculated according to the International Standard on dynamic light scattering, ISO 22412 [9].
| Surfactant | Critical Micelle Concentration (mM) | z-Average Diameter (nm) | Polydispersity Index |
|---|---|---|---|
| Triton XL-80N | 0.195 | 7.0 | 0.062 |
| Triton X-100 | 0.3 | 7.5 | 0.055 |
| Tween 20 | 0.059 | 8.5 | 0.211 |
| Tween 80 | 0.012 | 10.7 | 0.167 |
| Nonidet P40 | 0.25 | 15.4 | 0.207 |
The sensitivity of the NIBS optics incorporated in the Zetasizer Nano series enables easy size determination of surfactant micelles to be made without the need of a high-powered laser.
The critical micelle concentration of surfactants has been reported using various techniques such as conductivity, surface tension and fluorescence measurements [3-5].
Dynamic light scattering is a technique well suited for the determination of the cmc. In the results summarized here, measurements were made of different concentrations of triton X-100 prepared in deionized water. Below the cmc, the intensity of scattered light detected from each concentration was similar to that obtained from water. In addition, the autocorrelation functions obtained showed very poor signal to noise ratios i.e. very low intercepts and no size distribution information could be obtained from this data. However, once the cmc was reached, the intensity of scattered light increases due to the presence of micelles and the intercepts obtained in the correlation functions are much higher. Figure 2 shows the correlation functions obtained for the 0.05mM (below the cmc) and 0.6mM (above the cmc) concentrations of triton X-100 respectively.
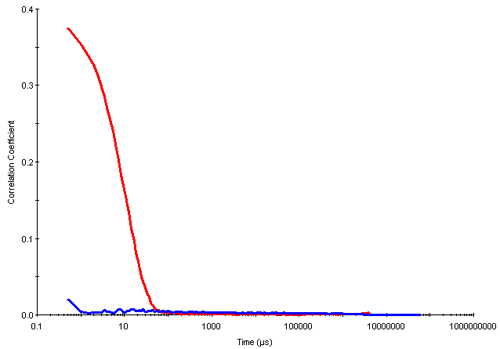
|
Figure 3 is a plot of the intensity of scattered light (in kilo counts per second) and micelle size (in nanometers) as a function of triton X-100 concentration (mM). The intensity data shows that the scattering detected for triton X-100 concentrations below the cmc are similar to that of deionized water. When the cmc is reached, the scattering intensity shows a linear increase with concentration. The intersection between the 2 lines at 0.25mM concentration corresponds to the cmc of triton X-100 which is in good agreement with literature values [10].
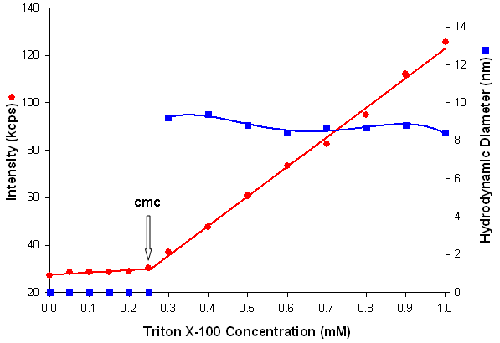
|
The aggregation number of a micelle is defined as the number of surfactant molecules per micelle and is often dependent upon the conditions of the disperse phase [11]. The aggregation number of a micelle can be determined if the molecular weights of the micelle and the surfactant monomer are known.
Determination of absolute molecular weight can be achieved through static light scattering techniques [12]. However, the preparation of samples for this technique can be time consuming. An estimate of molecular weight from DLS measurements can be determined using an empirical relationship between the hydrodynamic diameter and molecule/particle conformation. This molecular weight calculator has been incorporated into the Zetasizer Nano software.
A spherical particle, such as a triton X-100 micelle, with a hydrodynamic diameter of 7.5nm will give an estimated molecular weight of 72KDa. The average molecular weight of a triton X-100 monomer unit is 631Da [10]. Therefore, the aggregation number of a triton X-100 micelle is 114. This corresponds with similar values reported in the literature obtained by other techniques [13, 14].
Dynamic light scattering is an ideal technique for studying surfactant micelles as it is non-invasive and allows measurements to be made of the sample in its native environment.
The influence of surfactant concentration and dispersant conditions on the size and shape of surfactant micelles has been widely reported [15-17]. To illustrate the use of DLS in studying these effects, measurements were made on various concentrations of the ionic surfactant dodecyltrimethylammonium bromide (DTAB) in the presence of 0.1M sodium bromide. Figure 4 shows the z-average diameters (in nanometers) measured for a series of DTAB concentrations (in mM) prepared in 0.1M NaBr. There is a gradual decrease in the measured size as the surfactant concentration is increased. It can be seen from the sizes obtained, that the DLS technique is capable of monitoring changes of less than 1 nanometer.
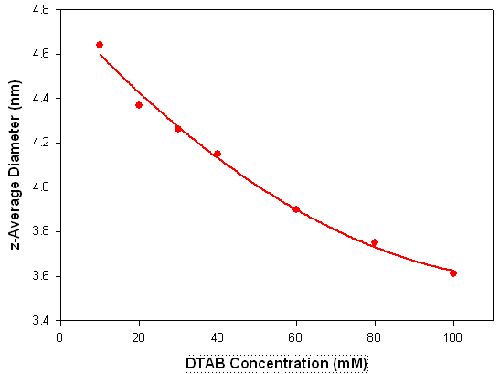
|
It has been reported that this decrease in micelle size with increasing surfactant concentration results from high charge repulsion forces between the DTAB micelles which increase the diffusion speed of the micelles[15]. This corresponds to a decrease in the hydrodynamic diameter.
Dynamic light scattering is an ideal technique for the characterization of surfactant micelles. The results reported here illustrate the application of DLS in the study of various aspects of micelle characterization.
[1] D.H. Everett, Basic Principles of Colloid Science (1988) The Royal Society of Chemistry, Cambridge, UK.
[2] Colloid Science: Principles, Methods and Applications [Ed. T. Cosgrove] (2005) Blackwell, Oxford, UK.
[3] K.S. Birdi, Handbook of Surface and Colloid Chemistry (1997), CRC Press, Boca Raton, FL.
[4] A. Dominguez, A. Fernandez, N. Gonzalez, E. Iglesias, and L. Montenegro (1997) J. Chem. Educ.74, 1227.
[5] Y. Nakahara, T. Kida, Y. Nakatsuji and M. Akashi (2005) Langmuir21, 6688.
[6] German patent 19725211
[7] US patent 6016195
[8] Japan patent 2911877
[9] International Standard ISO 22412:2008 Particle size analysis - Dynamic light scattering (DLS)
[10] Handbook of Industrial Surfactants [Compiled by M. Ash and I. Ash] (1993), Gower, UK.
[11] G.D.J. Phillies and J.E. Yambert (1996) Langmuir 12, 3431.
[12] K. Mattison and M. Kaszuba (2003) American Biotech. Lab. June
[13] C.J. Biaselle and D.B. Millar (1975) Biophys. Chem. 3, 355.
[14] P.J. Tummino and A. Gafni (1993) Biophys. J. 64, 1580.
[15] M. Pisarcik, F. Devinsky and E. Svajdlenka (1996) Colloids and Surfaces 119, 115.
[16] S. Ozeki and S. Ikeda (1982) J. Colloid. Int. Sci. 87, 424.
[17] V.K. Aswal and P.S. Goyal (2000) Physical Review 61, 2947.