Individual paper making plants vary a great deal depending on the product manufactured, e.g. tissue, kraft paper, fine paper etc.
Each of the products has its own particular requirements in terms of physical properties and these are achieved using a variety of different stocks and additives.
The interaction of all of the materials used on the physical properties of the product and the efficiency of the plant is complex, but there is a parameter that can be used to help - the zeta potential.
Zeta potential is the parameter that determines the electrostatic interactions between particles, a high value, positive or negative, prevents flocculation.
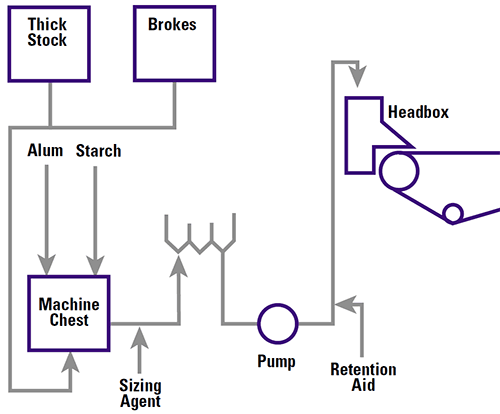
|
Reducing the value closer to zero allows particles to approach each other and flocculate. Changes to zeta potential can affect retention values, strength, pitch deposition, additive requirements and hence cost.
In addition to the process itself, effective effluent treatment is highly dependent on the zeta potential of material discharged. The Malvern Zetasizer makes the measurement of zeta potential simple, fast and reproducible.
The zeta potential of pulp and other particles in the process may vary for a number of reasons, e.g. changes in refining, pH, pulp source, broke content and quantities of additives used.
Measurements of zeta potential can be used in two different ways.
Firstly, a zeta potential 'profile' can be made of plant when everything is working well. Problems that arise later can be investigated by looking at variation from this norm.
Secondly, existing processes can be improved by looking at the zeta potential at each stage of the process.
At a fine paper mill, typical ash retention reduced from 35% to 25%. All the usual parameters were investigated, basis weight, speed, pH, additives and procedures, but none correlated with this problem. However, the head box zeta potential was rather high at about +9mV and increased occasionally to +17mV. This related to a particular brand of starch which was interchanged at random. Not only could this problem be eliminated, but the use of a third starch reduced zeta potential further and increased ash retention to over 50%.
At another mill optimum conditions in the machine chest were about - 9mV and -5mV in the head box. The zeta potential of the broke varied from -5mV to +5mV on a daily time scale. (This meant the zeta potential in the head box varied). Knowing this enabled the stocks and additives to be varied to optimize retention.
The effluent from a de-inking plant was known to affect the effluent treatment system, but it was not known why. Alum and other proprietary chemicals were added, but the results were not always good, and the treatment costs were high. Zeta potential measurements showed that one effluent stream varied from 0mV to 60mV. This depended on the amount and type of deinking chemical used. This knowledge could be used to determine the optimum treatment conditions.
A mill producing brown sack-kraft had deposits on a guiding roller in the pressing section. At first sight, the low cationic demand and slightly negative value in the head box seemed acceptable. The Al3+ was however all complexed. Addition of extra Alum reduced the zeta potential to zero, the Alum was added before the mixing chest to prevent complexing with the starch and the result was increased retention of total dry matter, hence pigment and therefore increased whiteness.
Other benefits of this procedure included a 25% strength increase because of increased starch retention, better rosin retention, cure of foam problems and elimination of the silica retention aid as retentions were now so improved.
In an integrated mill, pitch deposits were a severe problem. Wood resins which occur as fine particles must be prevented from agglomeration to stop problems in the machine or final product.
In this case the head box zeta potential was still rather high at -6.0mV. The mill increased the dosing of alum to bring the zeta potential to zero and exchanged its point of addition with the starch which entirely cured the pitch problem.
A process may still be running with a negative zeta potential even after cationic chemicals are added.
This may be due to anionic starches, and though they are hardly ever used, they can originate from waste paper.
The answer is the addition of a high molecular weight low charge retention aid as well as Poly aluminium chloride to reduce the zeta potential of the pulp to nearly zero.
This can give a high increase in starch retention and the possibility of reducing costs by using more filler and cheaper fibre.
Measure cationic or anionic demand by titrating with electrolyte and noting the amount required to bring the zeta potential to zero.
The Malvern Zetasizer is an invaluable aid in studying the electrokinetics of pulp used in paper manufacture.
The interactions of fiber and particles in the pulp are important in determining the efficiency of the process.
The Zetasizer can be used for problem solving by enabling a complex process to be analyzed at each stage which is then more easily understood.