Dextran is a complex polysaccharide that is used in various fields such as the pharmaceutical, photographic, agricultural, and food industries. Glucose molecules (C6H12O6) are joined into chains of varying lengths with short (mostly one or two sugar units, less than 5%, see fig.1) side branches. Dextran may be synthesized from sucrose by bacteria (e.g. Leuconostoc mesenteroides streptococcus and Streptococcus mutans) and can also be produced by other bacteria and yeasts.
In this application note dextran is characterized using two light scattering techniques at one angle and one concentration. The size and molecular weight of polysaccharides are often used as standards for chromatography calibration.
|
This polysaccharide polymer exists in many chain lengths and various polymerization fractions of narrow molecular weight are commercially available. Dextran fractions are readily soluble in water and electrolyte solutions to form clear, stable solutions over a wide pH range and at high concentrations. Other solvents exist, notably methyl sulfide, formamide, ethylene glycol, and glycerol, but this polysaccharide is insoluble in monohydric alcohols (e.g. methanol, ethanol, isopropanol) and most ketones (e.g. acetone, 2- propanone).
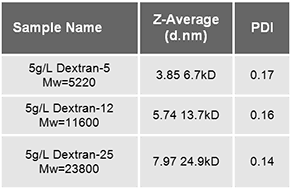
Three different molecular weight standards were prepared (Dextran-5, 12 and 25 from Pharmacosmos A/S) in deionized water at a concentration of 5 g/L. Samples were subsequently filtered through 0.1 µm pore size membranes (Whatman Anotop) without difficulty. The nominal molar mass of the fractions were provided as 5220, 11600, and 23800 g/mol.
Samples were measured in the Zetasizer Nano S by dynamic light scattering (DLS).
All sample solutions appeared clear to the eye without visible aggregation or tint confirming the dextran is well-dissolved. Even at the smallest size (Dextran-5) the light scattering setup is sensitive enough to provide significant scattering signal for analysis.
The dynamic light scattering technique (DLS) uses a laser to illuminate molecules diffusing under Brownian motion, and observes intensity fluctuations in the scattered light to determine the diffusion coefficient of the molecules, and from this the hydrodynamic size. For the mean size and polydispersity, this technique only requires knowledge of the viscosity of the solvent (water) and the temperature. The experimental result consists of a light scattering average size, the z-average, and a polydispersity index, PDI, obtained from a cumulant or single exponential fitting analysis (data are summarized in table 1).
|
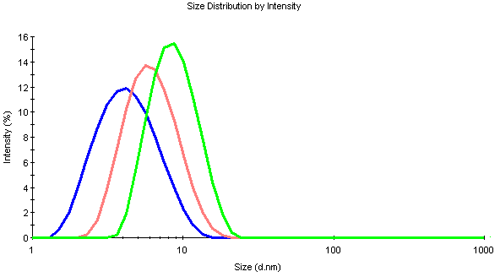
Distribution information may be obtained from a regularization without any assumption of the distribution shape. The size distributions by intensity are overlaid in figure 2.
|
The size distributions and z-average results may be used to estimate the molar mass from an empirical model for linear polysaccharides. The predicted values are listed in table 1.
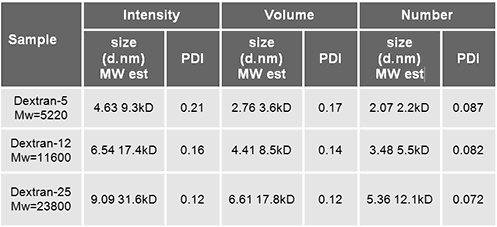
The distribution analysis for polymers is typically provided in terms of Mw/Mn. In light scattering the intensity is the measured quantity, and only indirectly through transformation of the result can a volume or even number distribution be obtained. Caution is generally advised since a rescaling with a cube of the diameter for volume and a sixth power of the diameter for number can significantly skew the resultant distribution towards small sizes: this should only be undertaken for high quality correlation function data. Since these dextrans are essentially GPC standards the transformations are justified.
For each distribution (intensity, volume, and number) a mean and a polydispersity index may be obtained from the software and these values are listed in table 2. The results show that the polydispersity index for the Dextran-25 is lowest which indicates that Dextrane-25 is the most monodisperse of the standards. This is confirmed from the specifications.
|
In order to compare the specifications given for these dextrans with the light scattering results the parameter Mw/Mn may be used. This is often called polydispersity in polymer chemistry. Here, the values for Mw/Mn of 1.64, 1.54, 1.47 (DLS) are in reasonable agreement with 1.60, 1.43, 1.30 (GPC) for Dextran-5, Dextran-12, and Dextran-25. The expected empirical dependence is:
PDI ~ ( Mw/Mn - 1 )2
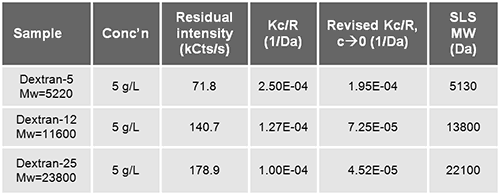
In static light scattering (SLS) the absolute MW is typically determined by detecting the scattered intensity from several known sample concentrations. Here, a single concentration method and prior knowledge of the second Virial coefficient of dextran in water (from Malvern Application Note MRK577-01: 0.011 mol mL / g2) is used: The measured intensity (from table 1) is adjusted for water (25Kcps/s), scaled with toluene (237.1 kcps), and Kc/R computed using dn/dc=0.150 mL/g #.
The molecular weights obtained from SLS (listed in table 3) are acceptably close to the nominal Mw from the manufacturer.
|
Dextran was characterized by dynamic and static light scattering to obtain molecular weight and polydispersity information. Results are in good agreement with sample specifications obtained from gel permeation chromatography.
# Refractive index increment data-book for polymer and biomolecular scientists, A. Theisen, C. Johann, M.P. Deacon, S.E. Harding, Nottingham Press (2000)