Polypropylene tacticity has a significant impact on polymer properties such as rigidity, flexibility, mechanical strength, melting temperature and optical transparency. The amorphous fraction (atactic) of the polpropylene is soluble in xylene; therefore the level of xylene soluble (XS) material in a polypropylene sample directly relates to its tacticity. Because of this relationship, the determination of the xylene soluble fraction is a critical measurement parameter during polypropylene manufacture.
ASTM D5492 [1] and ISO16152 [2] describe the gravimetric method that has historically been used to determine the amorphous (xylene soluble) portion of the polypropylene. This method is, however, both cumbersome and operator dependent. It requires a significant amount of solvent (as much as 400 mL/preparation) and can be inconsistent during the filtration and drying steps.
Alternative techniques such as bench-top spectroscopy instruments (NMR and NIR) have been used to replace the day-to-day gravimetric method especially in QC laboratories. Although NMR and NIR instruments eliminate the need for solvent and provide reduced analysis time, their results must be correlated with those from the conventional method for every type of catalysis used in the process. They have also proven to be polymer form (powder/pellet) dependent and can only measure within the correlation range. The use of these alternatives therefore does not eliminate the need for the conventional gravimetric xylene extraction as the reference method.
With its ability to accurately measure concentration and recovery, and handle solvents in an efficient and safe manner, gel-permeation chromatography (GPC) represents an excellent alternative to these methods. The addition of advanced detection such as light scattering and viscosity adds the ability to measure not only concentration but also molecular weight and intrinsic viscosity during the same experiment. Finally, the use of the 'flow injection polymer analysis' (FIPA) technique maximizes its sensitivity by reducing the level of separation that occurs.
In this application note, the FIPA-xylene solubles (FIPA-XS) method is described and xylene solubles content of different samples of ethylene propylene rubber is measured. The results are compared with the traditional gravimetric method.
GPC separates molecules according to their hydrodynamic size as they pass through a column filled with a porous gel matrix. Molecules that are too large for the column's pores elute at the exclusion volume while molecules that are smaller than all of the column's pores elute at the total permeation volume. Typically, solvent molecules from the injection elute at the total permeation volume whereas those from the sample elute earlier.
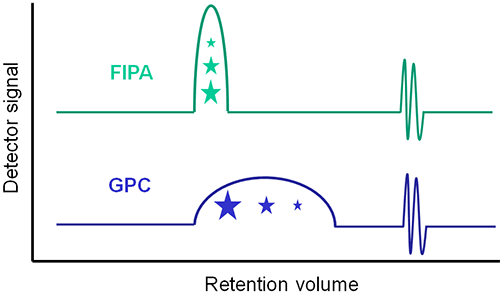
Flow injection polymer analysis (FIPA) can be thought of as a sub-set of the GPC technique. Using a FIPA column, the sample molecules are separated from the solvent which elutes separately but the sample molecules themselves elute together. This increases the sensitivity of the technique since the eluting peak will be narrower and taller, which is especially useful for very low molecular weight and low XS content samples. However, any results that are generated from the measurement will be average values as opposed to the full distribution that comes from GPC. Figure 1 illustrates the difference between FIPA and GPC.
FIPA is particularly useful for the measurement of xylene solubles because limited resolution is required to separate the xylene soluble fraction of the polymer from the xylene used to dissolve it. Furthermore, the use of GPC hardware significantly reduces the amount of solvent (in particular xylene) required, saving cost
|
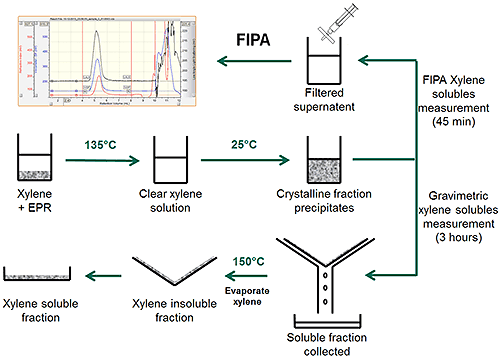
A full FIPA system is composed of a solvent delivery mechanism with an autosampler and a multi-detector module. The xylene solubles content, weight-average molecular weight and intrinsic viscosity of a sample are measured using refractive index, light scattering and viscosity detectors respectively in a single experiment. The use of a sensitive chromatography system means FIPA can measure xylene soluble content from 0.1% to 100% with excellent accuracy and repeatability, making it a clear choice for analysis of highly Isotactic polypropylene (PP) to ethylene-propylene rubber (EPR). FIPA can also be complemented with a semi-automated mini-extraction setup, SASP + Mini-Prep, for high throughput sample preparation and extraction which can prepare tens of samples for every analysis batch. In SASP, solutions are prepared in disposable 40 mL vials using a software-controlled automatic weighing and solvent dispensing setup which completely eliminates operator's involvement, solvent exposure and solvent handling. Figure 2 shows the TDAmax FIPA chromatography system with SASP.

|
The FIPA-XS extraction procedure closely follows the official gravimetric ASTM D5492 guidelines. Due to the high sensitivity nature of the FIPA instrumentation, the total sample mass and solvent volume requirements can be much reduced, resulting in a much more user friendly SASP + Mini-Prep procedure. Figure 3 shows a schematic of the extraction process. Initial dissolution of the sample and precipitation of the crystalline fraction follows the same procedure for both methods. In the gravimetric method, the sample is filtered, dried and weighed in a process that is prone to operator error. In the FIPA-XS method a small amount of the sample is filtered through a syringe filter and injected into the chromatography system. The removal of the drying step and the use of the auto-sampler significantly increases the repeatability and reproducibility of the measurement.
|
In the first experiment the xylene solubles content of a sample of EPR was extracted using the method described above. Duplicate injections of each sample were run on the FIPA-XS system and their recovery, molecular weight and intrinsic viscosity measured using the following conditions:
The results were compared to study the measurement reproducibility.
In the second experiment, five samples of different xylene solubles content were measured using the FIPA-XS method and the results were compared to a traditional gravimetric experiment.
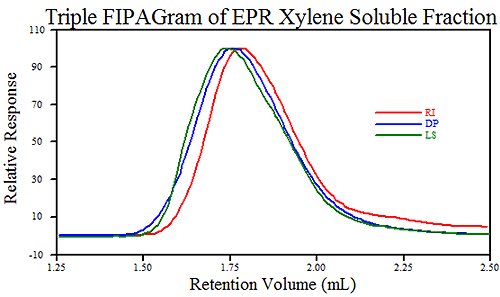
Figure 4 shows a typical FIPAgram of EPR.
|
Using Triple Detection, the FIPA technique can measure percent recovery, molecular weight (Mw) and intrinsic viscosity (IV) of the sample. If the concentration is the initial concentration of the polypropylene in the xylene, then the percent recovery directly represents the xylene soluble fraction. Table 1 shows the results from the six injections. The standard deviation and therefore the reproducibility of the experiment can be seen to be excellent.
| Sample ID | % recovery | Mw | IVw |
|---|---|---|---|
| 1 - Inj A | 15.20 | 299,700 | 2.391 |
| 1 - Inj B | 15.20 | 300,200 | 2.407 |
| 2 - Inj A | 15.34 | 297,700 | 2,383 |
| 2 - Inj B | 15.34 | 299,700 | 2.401 |
| 3 - Inj A | 15.16 | 301,800 | 2.416 |
| 3 - Inj B | 15.36 | 297,100 | 2.365 |
| Ave | 15.27 | 299,367 | 2.394 |
| SD | 0.09 | 1,718 | 0.018 |
| RSD | 0.01 | 0.57% | 0.76% |
A critical requirement of any alternative XS method is comparable precision and accuracy when compared to the reference gravimetric values. Table 2 shows the repeatability and reproducibility of this technique as a set of 5 samples were analyzed by three different laboratory persons each preparing and injecting samples in duplicate and injecting samples into three different FIPA instruments.
| Samples | %XS | Samples | %XS | Samples | %XS | Samples | %XS | Samples | %XS |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 1.321 | B1 | 2.844 | C1 | 6.507 | D1 | 18.275 | E1 | 30.801 |
| A1 | 1.39 | B1 | 2.764 | C1 | 6.594 | D1 | 18.152 | E1 | 30.933 |
| A2 | 1.317 | B2 | 2.743 | C2 | 6.505 | D2 | 18.216 | E2 | 30.815 |
| A2 | 1.32 | B2 | 2.805 | C2 | 6.461 | D2 | 18.157 | E2 | 31.038 |
| A1 | 1.306 | B1 | 2.7 | C1 | 6.485 | D1 | 18.207 | E1 | 30.659 |
| A1 | 1.323 | B1 | 2.76 | C1 | 6.499 | D1 | 18.071 | E1 | 30.53 |
| A2 | 1.26 | B2 | 2.744 | C2 | 6.373 | D2 | 18.071 | E2 | 30.628 |
| A2 | 1.315 | B2 | 2.669 | C2 | 6.466 | D2 | 18.024 | E2 | 30.77 |
| A1 | 1.184 | B1 | 2.901 | C1 | 6.528 | D1 | 18.091 | E1 | 30.801 |
| A1 | 1.321 | B1 | 2.676 | C1 | 6.531 | D1 | 18.049 | E1 | 30.923 |
| A2 | 1.28 | B2 | 2.774 | C2 | 6.394 | D2 | 18.055 | E2 | 30.649 |
| A2 | 1.272 | B2 | 2.79 | C2 | 6.454 | D2 | 18.105 | E2 | 30.662 |
| Ave | 1.301 | Ave | 2.764 | Ave | 6.483 | Ave | 18.123 | Ave | 30.767 |
| SD | 0.049 | SD | 0.067 | SD | 0.06 | SD | 0.078 | SD | 0.148 |
| RSD | 3.79% | RSD | 2.42% | RSD | 0.93% | RSD | 0.43% | RSD | 0.48% |
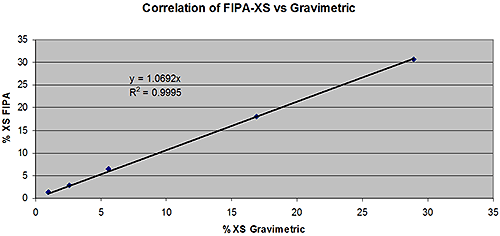
These results were compared to their label values based on the gravimetric method. The results are shown in figure 5. The linearity of the measurements is excellent with an R2 value of 0.9995. The fit line intersects the origin and the slope is very close to 1 showing that the FIPA-XS results are in excellent agreement with those from the gravimetric method over a wide range of xylene soluble contents.
|
FIPA employs advance detection technologies in a chromatography setup to provide not only xylene solubles but also critical molecular parameters such as the weight-average molecular weight and the intrinsic viscosity of the xylene solubles. This is very important to some polypropylene process technologies (e.g., Ethylene Propylene Rubber, EPR) which do require these additional parameters as process control measures.
The experiments described in this application note show how the FIPA-XS method can be used as an alternative technique to measure the xylene solubles content from samples such as PP and EPR. The results can be seen to be highly reproducible and to compare very well with the traditional gravimetric technique.