Cyclosporines are immunosuppressant drugs which are often delivered for ophthalmic indications in the form of microemulsions, offering thermodynamic stability, phase transition to a liquid-crystal state, very low surface tension and small droplet size. These features assist ocular absorption and retention of the drug, parameters upon which its efficacy and duration of action depends.
The formulation of ophthalmic emulsions (eye drops) with long shelf life is complicated by the tendency of emulsion droplets to aggregate, particularly in the presence of the polymers that are used to increase formulation stability.
This application note describes a comprehensive multi-parameter strategy for the characterization of microemulsions, designed to improve the efficiency of the approval process for both innovator and generic ophthalmic emulsions.
Please login or register for free to read more.
Cyclosporines are immunosuppressant drugs which are often delivered for ophthalmic indications in the form of microemulsions, offering thermodynamic stability, phase transition to a liquid-crystal state, very low surface tension and small droplet size. These features assist ocular absorption and retention of the drug, parameters upon which its efficacy and duration of action depends.
The formulation of ophthalmic emulsions (eye drops) with long shelf life is complicated by the tendency of emulsion droplets to aggregate, particularly in the presence of the polymers that are used to increase formulation stability.
This application note describes a comprehensive multi-parameter strategy for the characterization of microemulsions, designed to improve the efficiency of the approval process for both innovator and generic ophthalmic emulsions.
A Malvern Zetasizer Nano ZSP was used to measure the zeta potential, using electrophoretic light scattering (ELS), of two cyclosporine microemulsion samples (sample A, an innovator drug, and sample B, a generic drug), diluted 1:4 as per FDA guidance[1]. Zeta potential measurements assess microstructural stability, increased intermolecular repulsion meaning that formulations with larger zeta potential values are more electrostatically stable.
Size and polydispersity index (PDI) were also measured on the Zetasizer, using dynamic light scattering (DLS). Though such measurements are quick, simple, information-rich and require low sample volume, microemulsions often contain larger particles that are difficult to characterize using DLS. For this reason, use of laser diffraction as a complementary technique to calculate SPAN or D50 is advised.
Laser diffraction measurements were carried out using a Malvern Mastersizer 3000 with the Hydro SV small volume wet sample dispersion unit. The Hydro SV accessory dramatically reduces the sample requirements of laser diffraction, with only 5 mL of dispersant and 0.45 mL of sample required for the cyclosporine measurements presented here.
Finally, in order to fully account for the dilution involved in the laser diffraction and zeta potential measurements described above, and to provide rheological characterization as per FDA guidance[1], viscosity analysis was performed using Malvern’s Kinexus rheometer. Viscosity profiling gives an understanding of stability in terms of bulk flow (for instance, the likelihood of larger particles sedimenting), and allows quality and comparability assessments of samples under formulation conditions (without dilution).
Table 1 shows that both formulations have highly negative zeta potential values, suggesting that sample A and sample B are both electrostatically stable (although the zeta potential measured for the innovator is twice as great as that measured for the generic). A larger polydispersity index (PDI) was measured for sample A than for sample B. DLS analysis suggested the presence of large particles in both samples which would be better characterized using laser diffraction analysis.
| Parameter measured | Sample A | Sample B |
|---|---|---|
| Zeta potential (mV) | -60.4 +/- 0.8 | -30.1 +/- 0.2 |
| Polydispersity index (PDI) | 0.549 +/- 0.006 | 0.249 +/-0.011 |
Figure 1 shows analysis of the innovator (sample A) and generic (sample B) cyclosporine products using the Mastersizer 3000. Repeatability of D50 (and also D10 and D90) for each sample is excellent, confirming the measured differences in particle size distribution between the innovator and generic, with the innovator containing a higher number of >200 nm particles.
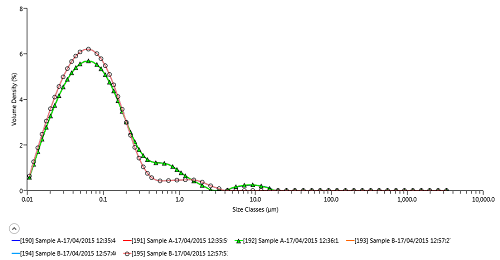
Figure 1: Laser diffraction particle size distribution analysis of innovator (A) and generic (B) cyclosporine microemulsions using Mastersizer 3000
| Record Number | Sample Name | Dx (10) um | Dx (50) um | Dx (90) um | |
|---|---|---|---|---|---|
| 190 | Sample A | 0.022 | 0.073 | 0.441 | |
| 191 | Sample A | 0.022 | 0.073 | 0.441 | |
| 192 | Sample A | 0.022 | 0.073 | 0.442 | |
| 193 | Sample B | 0.021 | 0.065 | 0.238 | |
| 194 | Sample B | 0.021 | 0.065 | 0.238 | |
| 195 | Sample B | 0.021 | 0.065 | 0.238 | |
| Mean | 0.021 | 0.069 | 0.340 | ||
| 1x Std Dev | 0.0005 | 0.005 | 0.111 | ||
| 1x RSD (%) | 2.251 | 6.515 | 32.708 |
Figure 2 shows flow curves for the innovator and generic samples, measured using the Malvern Kinexus. Both materials demonstrate a similar shear-thinning trend, with viscosity decreasing with increasing shear rate. Such a quality is important for an ophthalmic emulsion, with high viscosity in the absence of force giving the sample stability during storage and a relatively long shelf-life, and lower viscosity when force is applied, making application of the drug to the eye much easier. Despite the similarity of the flow curve trends, at any given shear rate the innovator is more viscous than the generic, suggesting that the likelihood of larger particles sedimenting in the generic microemulsion may be greater than in the innovator preparation.
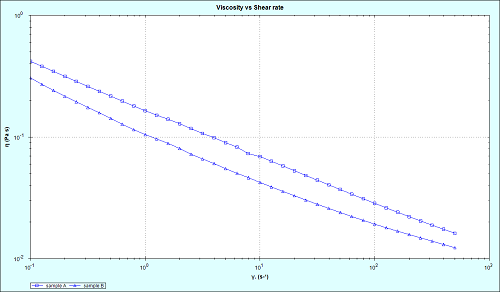
Figure 2: Flow curve analysis of innovator and generic cyclosporine microemulsions, using Kinexus rheometer
| Solution | |||
|---|---|---|---|
| Comparability measurement | Zetasizer | Mastersizer | Kinexus |
| Emulsion stability | X | X | |
| Polydispersity | X | X | |
| Particle size distribution | X | ||
| Ease of application | X | ||
By acquiring data using 3 complementary techniques, we were able to perform a comparability study of emulsion stability, polydispersity, particle size and ease of application of two cyclosporine microemulsions: the first an innovator preparation and the second a generic. The innovator cyclosporine was more stable than the generic in terms of both electrostatic (probed using the Zetasizer Nano ZSP) and sedimentation (assessed using Kinexus) stability. Despite this, the generic showed greater uniformity (lower PDI), the innovator containing more >200 nm particles and a significant quantity of particles >5 µm (as measured using the Mastersizer 3000). The decision of regulatory authorities to give approval to a generic is dependent on how clinically relevant these differences are deemed to be.
In addition to providing an assessment of innovator-generic comparability without prior dilution, Malvern’s Kinexus system offers many other ways to characterize microemulsions, often with real-life scenario applications. For instance, the effect of the blinking of a patient’s eye during application on the flow characteristics of the drug can be simulated by measuring using an appropriate shear rate range. Such data would give information on the ease of application of the drug, a quality that is of concern to developers due to the difficulties of creating microemulsion formulations that fully permeate the eye.
In summary, this application note demonstrates the importance of an orthogonal approach to therapeutic microemulsion characterization, with comprehensive assessment of characteristics such as shelf-life and size distribution requiring multiple technologies.
1) FDA-2007-D-0369: Draft Bioequivalence Guidance for Cyclosporine Ophthalmic Emulsion, 0.05%. U.S. Food and Drug Administration; recommended Jun 2013; Revised 2016.
2) C. Gooddeeris, F. Cuppo, H. Reynaers, W.G. Bouwman, G.Van den Mooter, Light scattering measurements on microemulsions: Estimation of droplet sizes. Pharmaceutical Nanotechnology, Int. Journal of Pharmaceutics 312, 187-195 (2006).
3) R. Daniels, Galenic principles of modern skin care products. Skin Care ForumIssue 25 (2001).
4) Z. Rahman, X. Xu, U. Katragadda, Y. S. R. Krishnaiah, L. Yu, M. A. Khan, Quality by design approach for understanding the critical quality attributes of cyclosporine ophthalmic emulsion. Mol. Pharm. 11 (3),787-799 (2014).
5) R. Lapasin, M. Grassi, N. Coceani, Effects of polymer addition on the rheology of o/w microemulsions. Rheologica Acta 40, Issue 2, 185-192 (2001).
6) R. Mafi, C. Gray, R. Pelton, H. Ketelson, J. Davis, On formulating ophthalmic emulsions. Colloids Surf B Biointerfaces 122, 7-11 (2014).
7) R. R. Hegde, A. Verma, A. Ghosh, Microemulsion: New Insights into the Ocular Drug Delivery. ISRN Pharmaceutics 2013, 1-10 (2013).
8) I. Danielsson, B.Lindman, The definition of microemulsion. Colloid Surf. 3, 391–392 (1981).
9) P. E. Petrochenko, M. S. Absar, Y. Wu, S. Y. Wong, S. Choi, J. Zheng, Characterization of the Globule Size Distribution of Cyclosporine Ophthalmic Emulsion to Establish Bioequivalence of a Test Formulation, U.S. Food and Drug Administration; presented at AAPS, Orlando, FL (October 25-29, 2015).