Using the Aeris Research edition, we were able to solve the crystal structure of AgCaVO4 from powder X-ray data in combination with the HighScore software suite. This demonstrates the high data quality which can be achieved with this PANalytical benchtop powder diffractometer.
We investigated the crystal structures of some of these silver vanadates using the Aeris Research edition.
The crystal chemistry of AIBIIXO4 (AI = alkali ion, BII = alkali- earth ion, X = P, V, As) is very rich and leads to numerous polymorphic phases which belong to 8 different structures types: olivine (e. g. LiMnPO4), arcanite (β-K2SO4), glaserite, tridymite, α-K2SO4, β-Na2SO4 and γ-Na2SO4 [1]. The phosphates have been widely investigated due to their ferroelectric and ferroelastic properties. Besides, more and more research is being carried out on this family of materials for possible applications as phosphors for LEDs [2]. Within this crystal chemistry, the vanadate compounds remain poorly investigated and in particular the silver based materials. With this in mind, we started to investigate the crystal structures of some of these silver vanadates using the Aeris Research edition.
The synthesis of AgCaVO4 was done in 2 consecutive steps. In the first step, we synthesized AgVO3 from Ag2O and V2O5 which were treated at 500°C for 24h. In a second step, AgVO3 was reacted with CaCO3 overnight at 450°C. Phase purity was checked by searching for the presence of the starting materials or their solid solutions. Pure phase was confirmed already after one single heat treatment at 450°C using the Aeris Research edition. Further analysis was carried out in order to solve the structure of this material. A 24 h measurement was recorded by repetitive scans of 45 minutes in the range 2θ = 14-140°.
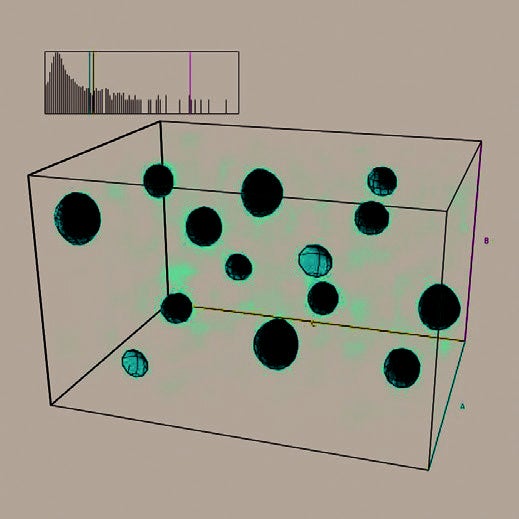
Figure 1. Fourier map obtained from Superflip as implemented in the HighScore suite locating the atoms of AgCaVO4 using the data collected with the Aeris Research edition.
All steps necessary to solve the structure of AgCaVO4 were carried out using the HighScore suite. After a peak search, an orthorhombic unit cell was found using Dicvol with a ≈ 7.03 Å, b ≈ 5.87 Å and c ≈ 9.38 Å. The space group determination was done using the program ExtSym [3] after an intensity Pawley extraction method. The structural model was found using Superflip [4] as illustrated in Figure 1. The final Rietveld refinement was carried out using the HighScore suite as shown in Figure 2. The corresponding atomic coordinates are shown in Table 1.
The final crystal structure is shown in Figure 3. AgCaVO4 crystallizes in the space group Pnma with cell parameters a = 7.03255(3) Å, b = 5.86684(2) Å and c = 9.38560(4) Å. This compound is made of one-dimensional chains of CaO6 octahedra running along the a axis which are interconnected by VO4 tetrahedra. The silver atoms are located in the voids of the structure. AgCaVO4 exhibits the arcanite structural type and is thus related to β-K2SO4. One can notice that not only a high precision is obtained on the heavy atoms positions but also on the lighter atoms such as the oxygen atoms.
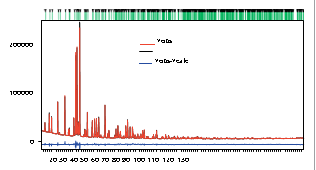
Figure 2. Rietveld refinement of AgCaVO4 after structure solution using Superflip.
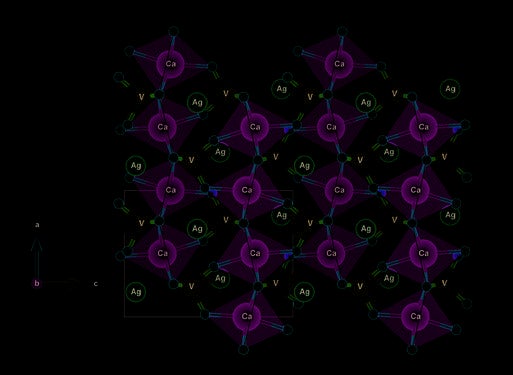
Figure 3. Crystal structure of AgCaVO4 projected in the ac plane. The Ca atoms are represented in green, the V atoms in blue, the Ag atoms in pink and the O atoms in red.
Table 1. Atomic coordinates obtained from the Rietveld refinement for AgCaVO4.
| Atom | Wyck. | x | y | z |
| Ag | 4c | 0.19623(6) | 0.250000 | 0.06801(5) |
| V | 4c | 0.2361(1) | 0.250000 | 0.3986(1) |
| Ca | 4c | -0.0003(1) | 0.250000 | 0.7256(1) |
| O1 | 4c | 0.5179(5) | 0.250000 | 0.0258(4) |
| 02 | 8d | 0.2401(3) | 0.5094(3) | 0.7935(2) |
| 03 | 4c | 0.3998(4) | 0.250000 | 0.5292(4) |
Using the Aeris Research edition, we were able to solve the crystal structure of AgCaVO4 from powder X-ray data in combination with the HighScore software suite. This demonstrates the high data quality which can be achieved with this PANalytical benchtop powder diffractometer.
Structure solution from an X-ray powder pattern requires high-quality data in order to be able to find a meaningful structural model. With the Aeris Research edition, you can not only do phase identification to monitor the progress of your chemical reactions but also collect high-quality data enabling you to solve a crystal structure. This has been demonstrated here with the example of the new compound AgCaVO4.