What is Zeta Potential? -1: Introduction to Zeta Potential Measurement
Introduction
Zeta potential is a physical property that represents all particles in a suspension. It can also clearly demonstrate the behavior of suspensions and emulsions. Understanding zeta potential reduces the time to create trial formulations. It also predicts long-term stability.
Colloid Science
The three fundamental states of matter are solid, liquid, and gas. If one state is finely dispersed in another state, it is called a colloidal system. These substances exhibit special properties with immense practical importance.
A colloidal system presents various examples, including aerosols, emulsions, colloidal suspensions, and association colloids. In certain conditions, particles in the dispersion combine with other particles to form aggregates, increasing in size, and are subjected to the influence of gravity.
The initially formed aggregates are called flocs, and their formation process is called flocculation.
Flocs may or may not undergo sedimentation or phase separation.These aggregates, when formed with increasing density, lead to coagulation.
Particulate binding occurs when the density is higher than the medium, leading to sedimentation, and when lower, forming cream by separation. This process of aggregation and coagulation can sometimes interchange.
Usually, the coagulation reaction is irreversible, differing from the reversible deflocculation seen in aggregation.
Figure 1 illustrates this process.

Colloidal Stability and DVLO Theory
In 1940, scientists Derjaguin, Verwey, Landau, and Overbeek developed a theory related to stability within colloidal systems. DVLO theory proposes that particle stability in a solution depends on the total energy function of particles, shown by ![]() .
.
![]() is determined by several contributions below.
is determined by several contributions below.
![]()
![]() is the potential energy due to solvents, and it’s the least contributing term to the separation of some nanoparticles involved in total potential energy. More important are the balances between
is the potential energy due to solvents, and it’s the least contributing term to the separation of some nanoparticles involved in total potential energy. More important are the balances between ![]() and
and ![]() , representing attractive and repulsive forces. Potentially,
, representing attractive and repulsive forces. Potentially, ![]() affects larger and more distant interactions.
affects larger and more distant interactions.
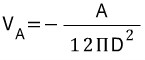
is shown where A is the Hamaker constant and D indicates particle separation.
The potential describing repulsion ![]() has a more complex equation.
has a more complex equation.
![]()
a represents the particle radius, π is the solubility of the solvent, κ is ion composition, and ζ is a function representing the zeta potential.

DVLO theory suggests that stability in colloidal systems is determined by the sum of van der Waals’ attractive forces and the repulsive forces from the electric double layer present during Brownian motion between particles.
This theory presents an energy barrier preventing two particles from coming close, balancing repulsive and attractive forces.
However, particles failing to overcome this energy barrier are irreversibly bound by attractive forces. Therefore, if particles have sufficiently high repulsive forces, dispersion will remain unaggregated, stabilizing the colloidal system.
If repulsion mechanisms are absent, aggregation or coagulation may lead to eventual sedimentation.

If zeta potential decreases (in cases of high salt concentration), there is a possibility of generating a “second minimum” where weak and potentially reversible bonds exist between particles.
These weak flocs are stable enough not to be broken by Brownian motion, but when external forces like forced stirring are applied, dispersion occurs.
Therefore, maintaining the stability of a colloidal system requires dominant repulsive forces. How can we achieve the stability of a colloidal system?
There are two fundamental mechanisms affecting the stability of dispersions.
Steric repulsion – This involves polymers that adsorb to particle surfaces, preventing closer approach, maintaining enough separation to defeat van der Waals forces.

Electrostatic or charge stability – This is an effect of particle interaction due to the charge distribution within the system. Each mechanism provides advantages suitable for the systems they’re part of. Steric stability is possible with simple and suitable polymers.
However, polymers can be expensive, and in cases like ceramic slips, mismatched mechanisms occur because polymers will burn off during casting and sintering, leading to shrinkage and defects. Electrostatic or charge stability has advantages for stabilizing or aggregating systems by merely changing the density of ions within a simple system.
This is a reversible process and not costly. Zeta potential has long been known as a good indicator of the degree of interaction between colloidal particles, and zeta potential measurement has been commonly used to check the stability of colloidal systems.
Surface Charge
Most colloidal dispersions in aqueous media carry charge. Many causes of surface charge depend on the intrinsic properties of particles and their surrounding environment, but we will consider the more important mechanisms.
Ionization of Surface Groups
Disintegration of acid groups on the particle surface leads to a negative surface charge, while the opposite process results in a positive base group charge due to disintegration by particle surface bases.
This article may have been translated automatically
{{ product.product_name }}
{{ product.product_strapline }}
{{ product.product_lede }}
