Overview
The Micromeritics BreakThrough Analyzer is a flexible gas delivery and management system for the precise characterization of adsorbent performance under process-relevant conditions. It delivers reliable adsorption data for gas/vapor mixtures using a flow-through system.
A safe and highly optimized device for collecting both transient and equilibrium adsorption data for multi-component systems. The BreakThrough Analyzer can be configured with up to six precision mass flow controllers and patented high-performance blending valves, delivering unparalleled flexibility in experimental design. The superior gas-delivery design ensures the precise control of both composition and flow rate, while minimizing dead volume.
The high-quality, stainless-steel column can hold 0.05 to 2.5 grams of adsorbent. Automated sample activation up to 1050 °C is possible with the precise, rugged, and reliable resistance furnace.
Operating pressures are controlled from atmospheric to 30 bar via a servo-positioned controlled valve. The thermostated environmental chamber delivers uniform temperature control of the entire system up to 200 °C, eliminating cold spots. The BreakThrough Analyzer secure door lock system ensures operator safety throughout the analysis.
Vapor generators can be added to the BreakThrough Analyzer to enable the use of important probe molecules such as water for experimental studies. The BreakThrough Analyzer easily connects to commercially available Fourier Transform Infrared and Mass Spectrometer systems for gas identification and quantification.

Features
-
Thermostated environmental chamber prevents condensation of vapor streams
-
Fully automated experimental design allows for easy experimental setup
-
Touch screen allows for easy instrument operation and monitoring of experimental conditions
-
Proprietary blending valves provide remarkable advantages for gas mixing and minimization of system dead volume
-
Up to 6 gas inlets and 2 vapor sources offer a wide range of analysis options with exceptional flow control and blending of multiple gases
-
Automatic door lock ensures temperature stability during analysis and user safety
-
Addition of detectors and other optional accessories: system scalability enables the expasion of capabilities over time through addition of detectors and other optional accessories (e.g. mass spectrometer, GC/MS, additional vapor sources, vacuum activation, others available upon request)
-
Column furnace: rugged, resistance furnace with high temperature capabilities up to 1050 °C
-
Electropolished 316 SS sample column with a capacity of up to 2.5g and is suitable for use with powders, other diameters are available for pellets or extrudates

Breakthrough adsorption dynamic analysis
Breakthrough analysis is a powerful technique for determining the adsorption capacity of an adsorbent under flow conditions. Dynamic breakthrough adsorption provides many advantages over static adsorption measurements.
- Easily collect multicomponent adsorption data
- Determine adsorbate selectivity
- Replicate process conditions
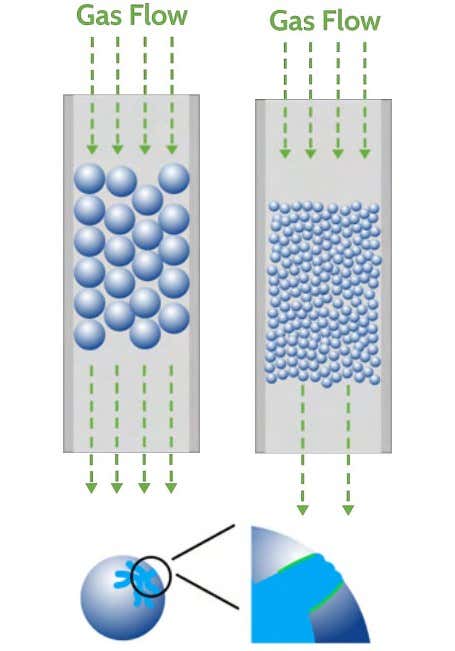
When conducting breakthrough analysis, sample preparation is a critical step in the analysis process to prevent pressure drop and mass transfer limitations.
- Pressure drop occurs when the interstitial space between particles is too small to accommodate the flow rate of gas.
- Mass transfer limitations occur when the pore size of the material is similar to the kinetic diameter of the adsorbate.
Appropriately sizing particles is therefore critical to obtain the best results.
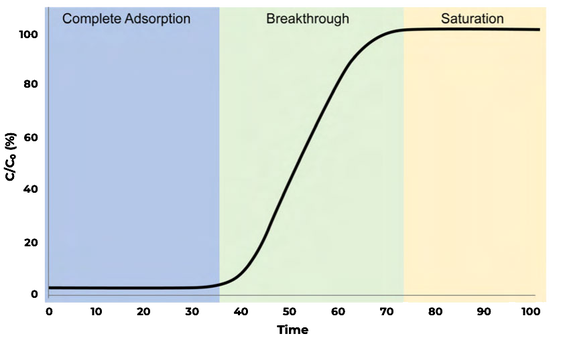
Examining a breakthrough curve
- Complete adsorption
The adsorbent completely adsorbs the adsorbate gas such that none can be detected at the outlet of the breakthrough column
- Breakthrough
The adsorbate gas is first detected at the outlet of the breakthrough column. Gas continues to adsorb; however, the adsorbent is no longer able to adsorb the entirety of the gas that is entering the breakthrough column
- Saturation
The adsorbent has reached saturation and can no longer adsorb the adsorbate gas, allowing it to pass through the column freely
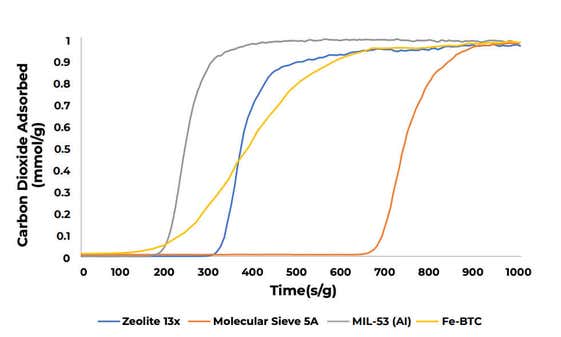
Carbon dioxide adsorption
Single-component carbon dioxide breakthrough adsorption experiments were conducted on zeolites 13X and 5A, and metal-organic frameworks MIL-53(Al) and Fe-BTC.
All materials were analyzed at 30 °C while flowing an equimolar gas stream consisting of 10 sccm nitrogen and 10 sccm carbon dioxide. A 1 sccm stream of helium was also blended into the feed gas stream as a tracer gas to aid in identifying the start of the breakthrough experiment.
The breakthrough curves for the four materials are plotted below on a mass-normalized axis. The total quantity of CO2 adsorbed follows the trend: molecular sieve 5A > zeolite 13X > Fe-BTC > MIL-53(Al).
The table below shows the total quantity adsorbed in mmol/g.
| Material | Material carbon dioxide adsorbed |
|---|---|
| ZEOLITE 13X | 2.94 |
| MOLECULAR SIEVE 5A | 3.52 |
| MIL-53 (AI) | 1.23 |
| FE-BTC | 2.30 |
Applications
-
- Natural gas separation
-
Natural gas is a mixture of hydrocarbons and other gases that must be purified prior to use in industrial applications and households for heating and food preparation.
- Direct air capture
-
DAC is difficult due to low concentrations of carbon dioxide in air along with other impurities including moisture, and the captured CO2 may be sequestered underground, sold, or converted into value-added chemicals to offset carbon emissions.
- CO2 adsorption
-
Power generation, chemical plants, and refineries are significant point sources for carbon dioxide emissions and the higher concentrations often require different operating conditions when compared to direct air capture.
- Olefin / paraffin separatons
-
Are a core part of the petrochemical industry and used to in the production of polymers such as polyethylene and polypropylene; these separations are energy intensive and increase CO2 emissions.
- Toxic gas adsorption
-
Porous solids are used for personal protection and also under development for the capture of toxic gases including sulfur dioxide, hydrogen sulfide, and nitrogen dioxide from natural gas or other process feeds.
- Water adsorption
-
Harvesting water from the air may be a critical technology for many parts of the world clean, where the fresh water supply is limited due to an arid climate or the increasing usage of water for agriculture.
- Zeolites
-
Pressure swing adsorption using Zeolite 5A, 13X, or LiX, which have high selectivity for adsorbing nitrogen are used commercially for air separation and producing oxygen.
- Silicas
-
Amine functionalized silicas are effective and highly selective adsorbents and used for the direct air capture (DAC) of CO2.
- Porous membranes / monoliths
-
Porous membranes and monoliths coated zeolites or MOFs are commonly used to improve the operational efficiency of separation processes.
- Activated carbon
-
Volatile organic component (VOC) from automobile fuel systems are captured by canisters filled activated carbon and these VOC emissions are minimized.
- Porous aluminas
-
Alumina – Supported Ionic Liquids are effective adsorbents with potential applications for the separation of CO2 from natural gas.
- Metal-organic frameworks
-
MOFs are highly selective adsorbents which are effective for demanding commercial applications including alkanes & olefins, olefins & alkynes, DAC, CO2 & CH4.







![[Micromeritics - Breakthrough-Analyzer - Multi component vapor analysis graphs.jpg] Micromeritics - Breakthrough-Analyzer - Multi component vapor analysis graphs.jpg](https://dam.malvernpanalytical.com/12302f92-af5a-4d85-8f00-b2bf00b85c85/Micromeritics%20-%20Breakthrough-Analyzer%20-%20Multi%20component%20vapor%20analysis%20graphs_Original%20file.jpg)
![[Micromeritics - Breakthrough-Analyzer - safety - touch screen panel.jpg] Micromeritics - Breakthrough-Analyzer - safety - touch screen panel.jpg](https://dam.malvernpanalytical.com/3dc78d7a-a961-46bd-8347-b2bf00b85e10/Micromeritics%20-%20Breakthrough-Analyzer%20-%20safety%20-%20touch%20screen%20panel_Original%20file.jpg)
![[Micromeritics BreakThrough Analyzer - software screen desktop.png] Micromeritics BreakThrough Analyzer - software screen desktop.png](https://dam.malvernpanalytical.com/301104fb-707e-4a26-87d2-b2bf00b85c0d/Micromeritics%20BreakThrough%20Analyzer%20-%20software%20screen%20desktop_Original%20file.png)
![[Micromeritics - Breakthrough-Analyzer - high pressure adsorption graphs.jpg] Micromeritics - Breakthrough-Analyzer - high pressure adsorption graphs.jpg](https://dam.malvernpanalytical.com/2663076f-e9ba-4d33-938b-b2bf00b85a42/Micromeritics%20-%20Breakthrough-Analyzer%20-%20high%20pressure%20adsorption%20graphs_Original%20file.jpg)
