The surface zeta potential cell is an accessory available for the Zetasizer Nano range of instrument that can be used to determine the zeta potential of flat surfaces. The surface zeta potential cell is supplied as part of a kit (ZEN1020) comprising of alignment and sample preparation tools (Figure 1).

|
The surface of interest is glued onto a sample holder which is inserted between the electrodes of the surface zeta potential dip cell. The cell is then immersed into a dispersant containing a suitable tracer particle contained in a square cuvette and inserted into a Zetasizer Nano instrument. The vertical position of the sample is moved with respect to the detection optics by an adjuster at the top of the cell.
The movement of the tracer particles upon the application of an electric field will be due to a combination of the particle's electrophoretic mobility and the electro-osmotic flow contribution from the wall. This motion can be described with a simple model allowing for the calculation of the surface zeta potential of the sample surface [1, 2].
Other application notes available from the Malvern website have discussed the determination of the surface zeta potential of silica and PTFE surfaces [3, 4] and also the influence of ionic strength [5]. This application note discusses the effect of temperature on surface zeta potential.
There have been few studies concerned with the relationship between surface zeta potential and temperature and there is poor agreement between published results [1, 6].
This application note discusses the effect of temperature on the surface zeta potential of a silica test plate.
The silica samples used in this study were cut from an uncoated microscope slide and the test plate sample was cut to 4mm width, the spacing of the Surface zeta potential cell electrodes, <8.0mm in length and were generally no more than 1.5mm in thickness. KCl, KOH and HCl were all obtained from Sigma-Aldrich (Dorset, UK). The tracer particles used were Coffee Compliment milk substitute obtained from Premier Foods UK in predispersed form.
The surface zeta potential of the silica was measured in 1mM KCl at pH7.0 +/-0.1 pH units.
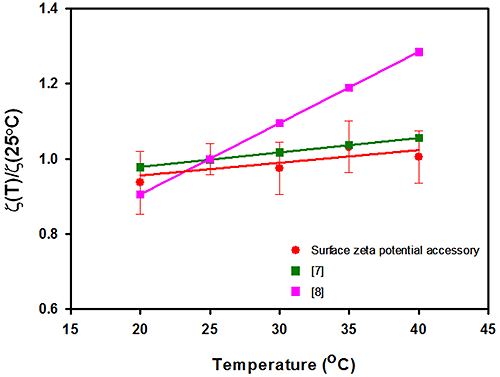
Figure 2 summarizes the surface zeta potential results obtained for the silica sample as a function of measurement temperature. The surface zeta potential data is normalized to the value recorded at 25°C and plotted against temperature. The graph also contains surface zeta potential values for silica obtained from other studies [7, 8].
The data obtained in this study predicts a slope of 0.34% change in surface zeta potential per °C. This is in excellent agreement with a recent study using capillary electrophoresis [7] who measured a slope of 0.39% per °C, but in poor agreement with published streaming potential results [8] which derived a relationship of 1.75% per °C.
The error bars obtained from repeat measurements in this study demonstrate that the technique is reproducible with temperature variation with an RSD varying from 5% to 10% and that no apparent increase in uncertainty exists with temperature. The whole cell (surface zeta potential cell, cuvette and dispersant) is immersed in the instrument's temperature controlled cell block and setting each temperature is therefore trivial in comparison to other techniques as the whole apparatus is at the same set temperature.
|
This application note summarizes the effect of temperature on the surface zeta potential of silica using the surface zeta potential cell accessory for the Zetasizer Nano. The data obtained in this study predicted a slope of 0.34% change in surface zeta potential per °C.