Ferritin is the iron (Fe) storage protein inside cells which forms a 24-mers. Molecular weight was larger than the expected 474 kDa, and hydrodynamic size of ~18nm correlated with this finding.
The charge was found to be negative.
The samples were kindly submitted by ApaClara Limited, UK
Life is built on the transport of oxygen and a key mineral involved in oxygen transport is iron. Ferritin is an iron storage protein present in mammalian cells. It is a spherical molecule that has a hollow interior that can bind iron atoms.
Nanotechnology is being used to investigate the possibilities of building nano-sized structures. This may be accomplished by utilizing ferritin to encapsulate metallic alloys inside ferritin for Nanotechnology and Medical Applications.
This application note presents a thorough characterization of (native) ferritin by light scattering to find its size, molecular weight, and zeta potential. These parameters may be used to control the stability and performance of non-native "nano" ferritin.
Native ferritin was prepared in deionized water at various concentrations. Samples were filtered through a 0.1 μm Whatman Anotop filter (cat. 6809-1012) and then measured in the Zetasizer Nano.
Samples appear slightly brown in color because of the oxidation state of iron in the ferritin molecule. The Helium Neon laser wavelength (633nm) in the Zetasizer Nano is not absorbed; enough light is scattered and transmitted to be able to perform experiments.
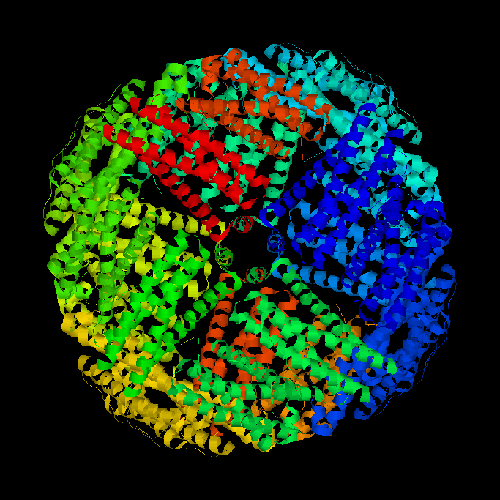
Ferritin complexes can be present in oligomeric forms. Native ferritin consists of a 24-mer of peptide subunits that are assembled into a hollow spherical shell (Figure 1). The expected molecular weight of the ferritin complex is 474,000 g/mol or 474 kDa.
|
In static light scattering (SLS) the absolute MW is determined by detecting the scattering signal from known concentrations of the sample. This measurement provides the average molecular weight of all species in solution.
The refractive index increment dn/dc of many proteins is approximately 0.185 mL/g. Due to its iron content ferritin behaves slightly differently. The dn/dc of ferritin was determined with a differential refractometer as being 0.158 mL/g (633nm, 22°C). The result of a dilution series of this sample showed a MW somewhat higher than expected: 1090 kDa.
The increased MW indicated that ferritin was not present solely in the monomeric form of 474 kDa, but on average was present as dimers.
The MW result was confirmed with dynamic light scattering (DLS). Here, the fluctuations in the scattered light are analyzed to detect the diffusion of the molecules and deduct their hydrodynamic size.
The sphere that is formed by ferritin is approximately 16 nm in diameter. This is a rather large protein, yet despite its unusual structure it follows the size predictions for globular proteins quite well.
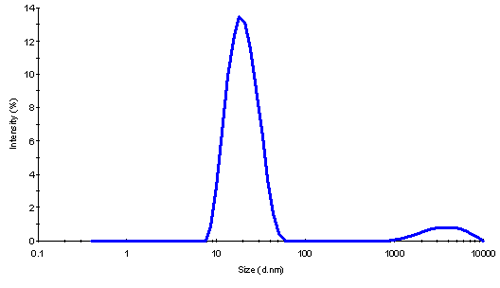
The z-average size and polydispersity index (PDI) measured by DLS provide a good indication of the state of the sample. A low polydispersity of PDI<0.1 usually indicates a highly monodisperse sample with only one oligomeric species present in solution. Higher PDI values are evidence of a broad distribution of different species in the sample. At a concentration of 1 mg/mL the native ferritin showed a large PDI, and it remained high on dilution. The results from DLS are summarized in Table 1.
| Native ferritin | z-average | PDI |
|---|---|---|
| 1 g/L | 17.8 nm | 0.29 |
| 0.4 g/L | 18.5 nm | 0.29 |
| 0.3 g/L | 19.2 nm | 0.25 |
| 0.2 g/L | 19.1 nm | 0.23 |
| 0.1 g/L | 20.4 nm | 0.25 |
| 0.05 g/L | 19.9 nm | 0.23 |
The size of globular proteins may be used to estimate their molecular weight. If this relationship is used for the concentration series, an estimated mw range from 560 kDa (for 17.8 nm) to 770 kDa (for 20.4 nm) clearly underscores that more than a single monomeric form of ferritin is present in solution. While a distribution analysis showed a small contribution of aggregates in this sample (shown in fig. 2), this was too small to explain the large PDI values seen here. The large polydispersity is therefore caused by the presence of oligomers.
|
When an electric field is applied to charged molecules illuminated by a laser, any movement due to electrophoresis will cause a Doppler shift in the frequency of the scattered light. This effect may be exploited to study their mobility and hence zeta potential, which is related to the charge of the molecules in solution.
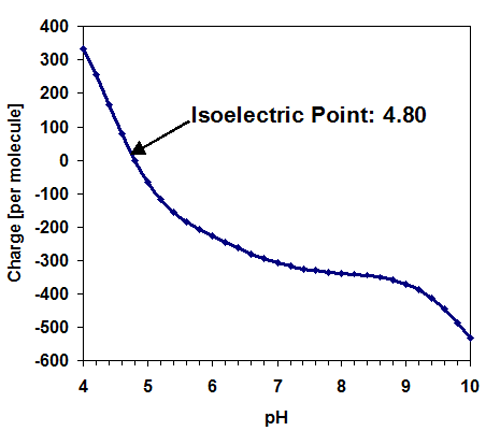
The molecular structure of ferritin is known and may be used to predict its charge in solution. Here, the sample was prepared at pH 7 and the estimated charge of the molecule was predicted as: -306, based on its sequence with a predicted isoelectric point at pI=4.80 resulting.1
|
The zeta potential is the charge induced potential at the slipping plane For a negatively charged molecule a negative zeta potential is expected. The results from two different concentrations are listed in the table below.
| Native ferritin | Zeta potential | Conductivity |
|---|---|---|
| 1 g/L | -34.6 mV | 0.043 mS/cm |
| 10 g/L | -32.3 mV | 0.288 mS/cm |
The results confirm the negative charge of the protein. Since the samples were prepared in distilled water, the conductivity measurement may be used to estimate the charge. A rough estimate assuming that all charges in the solution originate from the protein, and that these charges behave like Na+Cl- gives an estimated charge of 105 Electrons per molecule for the preparation at 1g/L and a protein charge of 85 Electrons at 10g/L. The charge is expected to be slightly lower at the higher concentration. Surprisingly, these estimates are in reasonable agreement with the negative charge expected from its sequence -306 (with the incorrect assumption that all titratable sites are considered isolated from each other and independent of the local environment. In addition, the effect of shielding by the electric double layer is not taken into account here. This would lead to a higher net charge than the one calculated ignoring the contribution from external ions that reduce the effective charge at the slipping plane).
Ferritin was characterized by the combination of three light scattering techniques. Ferritin is a protein that stores and releases iron in a controlled fashion and may be an ideal target for bio-nano-material production.
The size of ferritin and its interior is appropriate for nano-technology applications. The combination of the information of the size (obtained by DLS), the molecular weight (obtained by SLS) and the charge (obtained by ELS) may be employed to control and ultimately produce better nanotechnology products. In addition, its biological compatibility may allow its use for biomedical applications.
1 http://www.scripps.edu/~cdputnam/protcalc.html