Isothermal Titration Calorimetry (ITC) is a powerful and versatile technique that is widely used for measuring the binding affinity and thermodynamics of equilibrium association reactions. ITC is also an accepted, universal label-free assay technique that can be applied to any enzyme-catalyzed system, provided that the reaction is associated with a change in enthalpy – see the companion whitepaper and other review articles [1-8]. ITC does not rely on a spectroscopic readout and labelled reagents, and the experiments are quick – ITC data for a Michaelis-Menten curve can be generated in 30 -90 minutes with current instrumentation – and only small amounts of material are required, making it an appealing alternative for enzyme kinetics and inhibition assays.
Please login or register to read more
Isothermal Titration Calorimetry (ITC) is a powerful and versatile technique that is widely used for measuring the binding affinity and thermodynamics of equilibrium association reactions. ITC is also an accepted, universal label-free assay technique that can be applied to any enzyme-catalyzed system, provided that the reaction is associated with a change in enthalpy – see the companion whitepaper and other review articles [1-8]. ITC does not rely on a spectroscopic readout and labeled reagents, and the experiments are quick – ITC data for a Michaelis-Menten curve can be generated in 30 -90 minutes with current instrumentation – and only small amounts of material are required, making it an appealing alternative for enzyme kinetics and inhibition assays.
A typical experiment for enzyme kinetics has the enzyme in the ITC cell and substrate in the ITC syringe. It is necessary to accurately quantify the molar concentration of both enzyme and substrate. This is most conveniently done using spectroscopic means where the reactants have chromophores and experimentally determined or calculated molar extinction coefficients. Biochemists often express amounts of protein in terms of activity units, where 1 unit of an enzyme is defined as the amount that catalyzes the formation of 1 μmol of product per minute under defined conditions. If an enzyme preparation is impure, the concentration may be expressed as units per mL. The specific activity is defined as units per mg of protein; the greater the purity of the preparation, the greater the specific activity. With enzymatic assay by ITC, it is necessary to determine the apparent molar enthalpy, making it advantageous if the exact molar concentration of substrate can be determined in the solution as well as active enzyme concentration for accurate kcat determination. It is also helpful if there is some indication of approximate KM so that appropriate substrate concentrations can be used to yield a complete Michaelis-Menten curve with some points of the curve below KM and some above it.
Typical enzyme and substrate concentrations for ITC enzyme assays are in the range of spectroscopic assays. Enzyme in the ITC cell is in the sub-nanomolar to micromolar range (the higher the KM value, the higher the concentration needed). The required concentration is often less than that used for traditional ITC protein-ligand binding assays. Substrate in the syringe is in the micromolar to millimolar range and is higher than the KM value. See below for guidelines.
With all ITC experiments it is important that the reactants are made up in appropriate and well-defined buffer systems. Enzyme kinetics parameters will vary due to pH, salt, pH, etc. In order to avoid excessive heats of dilution it is necessary to dialyze the enzyme in the chosen buffer and then use the same buffer to make up the substrate solution. When choosing a buffer system, it might be necessary to prepare substrate solutions at quite high concentrations, needing a high concentration of buffer salt to ensure good buffering of the solution and prevent pH drift. As an added precaution, it is recommended to check the pH of all solutions and mixtures both before and after performing titrations. If the enzyme reaction requires any cofactors, the concentration of cofactor is matched in substrate and enzyme solutions.
There are two methods that can be used to measure enzyme kinetic parameters with ITC: the multiple-injection method, which uses pseudo-first order conditions, and the continuous assay/single injection method, which uses a single injection of substrate into enzyme. Descriptions for each method are given below. For more details on experimental design and data analysis, refer to References 2-10.
ITC experiments for enzyme kinetics reactions are commonly conducted this way, with multiple injections of the substrate generating multiple rate determinations under pseudo-first order steady-state conditions in a single experiment.
The rate data can be obtained by having relatively low amounts of enzyme in the cell, relatively high amounts of substrate in the injection syringe and by leaving shorter gaps between injections. The aim here is to ensure that subsequent to each injection, steady-state conditions are maintained and no more than 5% of the injected substrate is depleted prior to the next injection. In addition, it is also necessary a control where the identical solutions of substrate used in enzyme assays are injected into the reaction buffer.
To obtain a Michaelis-Menten plot from a multiple-injection ITC experiment, it is necessary to measure the total molar enthalpy of the enzymatic reaction (∆Happ). The method is described below.
Figure 1 shows ITC data for the titration of 43 mM 4MU-α-GlcNS (substrate) into a 2 μM solution of a lysosomal enzyme[11]. The top figure is the raw data collected with a MicroCal iTC200, also showing the pre-injection baseline and dQ/dT for the 1st, 2nd, and 3rd injections. The bottom figure is the Michaelis-Menten plot, fit with the multiple-injection enzyme kinetics model included in the PEAQ-ITC software. In a separate experiment, ∆Happ was determined to be -7 kcal/mol. Fit kinetics parameters were kcat = 1.83 s-1 and KM = 3.34 mM.
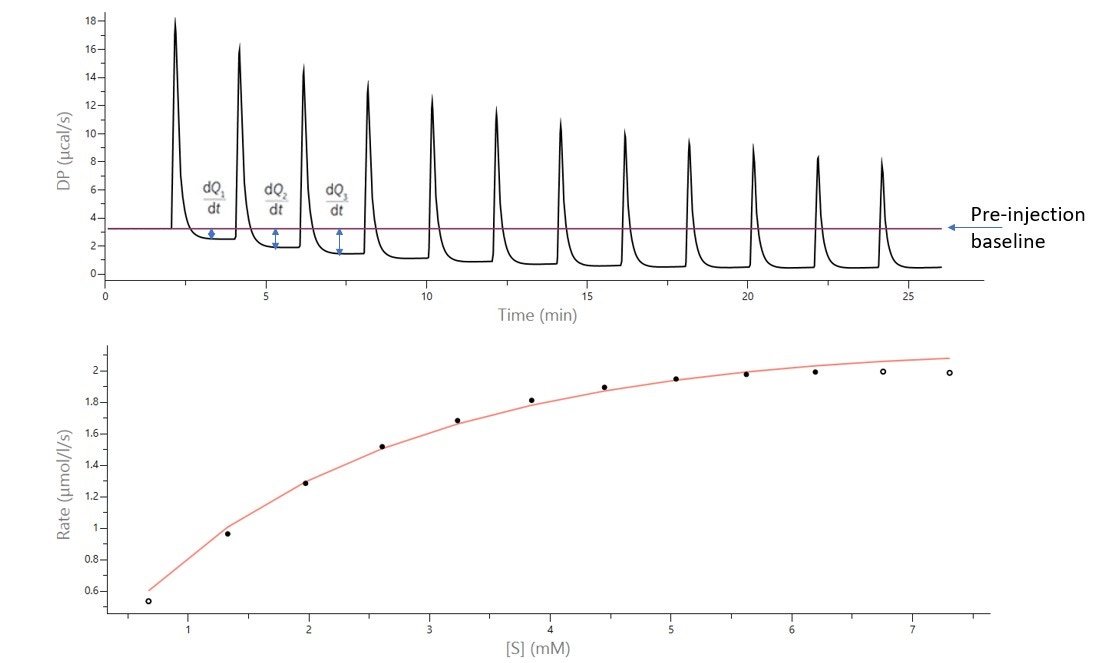
Figure 1: Raw ITC data (top) and fit data (bottom) for measurement of reaction rate for the hydrolysis of 4MU-α-GlcNS with a lysosomal enzyme. The top figure is the raw data collected with a MicroCal iTC200. There were 12 x 3.1 μL injections. The bottom figure is the Michaelis-Menten plot, fit with the multiple-injections enzyme kinetics model included in the PEAQ-ITC software. (Original data from reference 11)
Temperature: use typical temperatures for kinetics assays (20 °C - 37 °C).
KM greater than 10 μM: if lower than that, the continuous assay (below) is suggested.
Recommended enzyme concentration in ITC cell: 25 pM to 1 mM. In general, the tighter the enzyme-substrate affinity, the lower the enzyme concentration. Total enzyme concentration in the ITC cell should be much less than total substrate concentration in the syringe.
Recommended substrate concentration in ITC syringe: 10 μM to 100 mM, above KM and in excess of enzyme concentration in cell. As a general guideline, no more than 5% of substrate should be converted to product before the next injection. After several substrate injections, concentration of substrate in cell should not be above KM.
MicroCal PEAQ-ITC and iTC200: 1-4 μL per injection, 12-40 injections (total available volume of syringe is ca. 40 μL).
MicroCal VP-ITC: 3-15 μL per injection, 15-30 injections (total available volume of syringe is ca. 300 μL).
Suggested interval between injections: 2-3 minutes, to establish new thermal power baseline after each injection (Figure 1). Total time of experiment is 20-90 minutes, depending on the number of injections and interval between injections.
Using ITC data needs the determination of the enthalpy change, or ΔHapp, for the rate determination of enzyme and substrate reaction [1,2]. To measure ΔHapp by ITC, there must be enough enzyme in the cell to convert all injected substrate into product in a given time period, so baseline response returns to the same value after substrate injection as it was before injection. This experiment is similar to the continuous assay method (below). Integration of these peaks with respect to time yields the total heat produced by the reaction and dividing this total heat by the amount of substrate converted gives the total enthalpy change. See Figure 2 for an example.
Temperature: use typical temperatures for kinetics assays (20-37 °C).
Recommended enzyme concentration in ITC cell: 1 nM – 10 μM.
Recommended substrate concentration in ITC syringe: 10 μM to 100 mM, above KM and in excess of initial enzyme concentration.
Injection parameters: Injection(s) of 5 to 40 μL, followed by data collection for 300-2000 sec, or until thermal power baseline returns to pre-injection level (Figures 2 and 3).
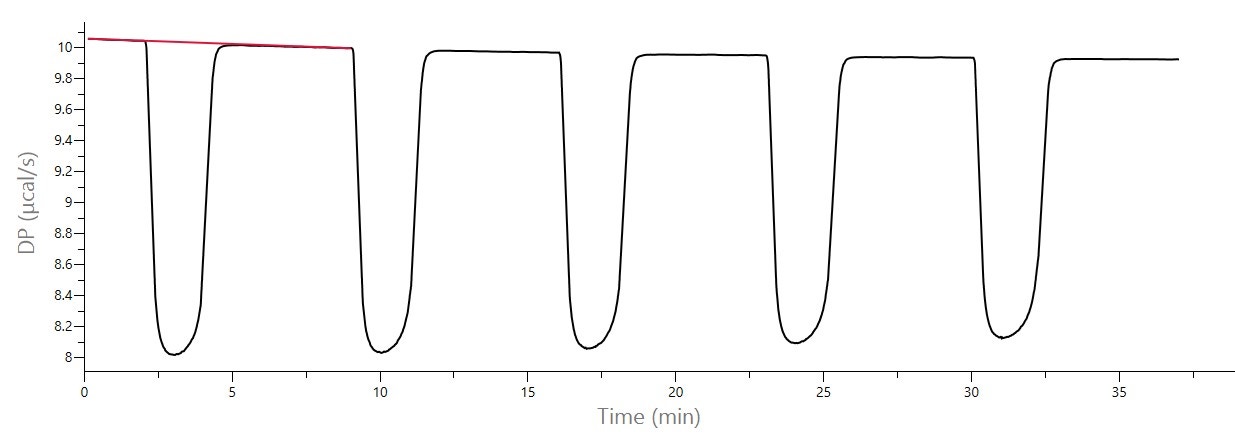
Figure 2: Raw ITC data for the hydrolysis of BAEE with trypsin, collected on a MicroCal PEAQ-ITC. Initial concentration of BAEE in syringe was 4 mM, initial concentration of trypsin in cell was 100 nM. There were 5 x 5 μL injections. The integration baseline (red) is shown for the first injection. MicroCal PEAQ-ITC software was used to calculate ΔHapp of -11.2 kcal/mol. (Data from Malvern Panalytical).
An alternative strategy for obtaining enzyme kinetic parameters involves continuous rate measurements after a single injection of substrate at concentrations higher than KM. In these experiments, thermal power is monitored as the substrate is completely depleted (Figure 3). At any given time, the reaction rate can be determined and the concentration of substrate at any given time can be calculated from the integral of the heat evolved [1,2]. Therefore, plots of rate vs. substrate concentration can be plotted to give a continuous kinetic curve.
An advantage to this method is that ΔHapp is determined from the same experiment, by integration of the area under the peak.
Temperature: use typical temperatures for kinetics assays (20 °C - 37 °C).
Recommended enzyme concentration in ITC cell: 1 nM -25 nM. In general, the tighter the enzyme-substrate affinity, the lower the enzyme concentration. Total enzyme concentration in the ITC cell should be much lower than total substrate concentration in the syringe.
Recommended substrate concentration in ITC syringe: 10 μM to 100 mM, above KM and in excess of enzyme.
Injection parameters: Injection(s) of 5 to 40 μL, followed by data collection for 300 sec - 2000 sec, or until thermal power baseline returns to pre-injection level. (Figures 2 and 3).
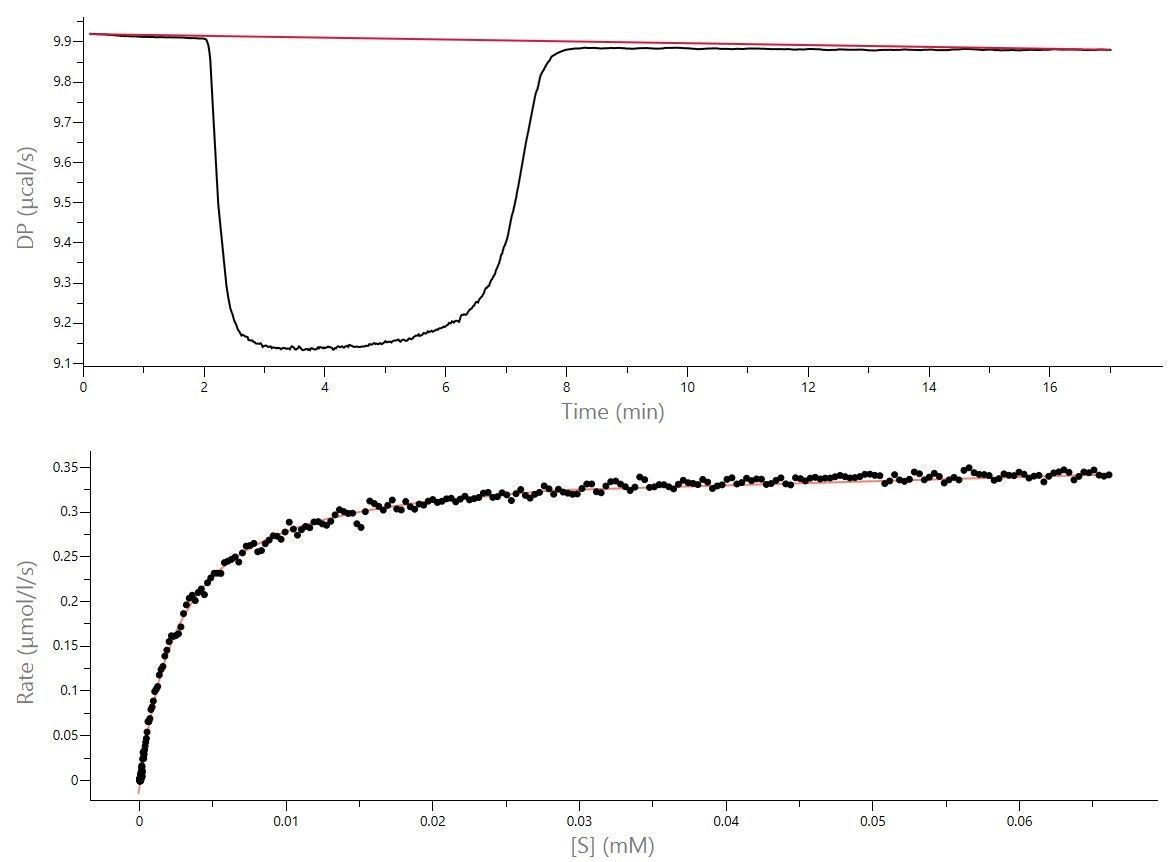
Figure 3: Raw ITC data (Top) and fit data (bottom) for the hydrolysis of BAEE with trypsin, collected on a MicroCal PEAQ-ITC. Initial concentration of BAEE in syringe was 4 mM, initial concentration of trypsin in cell was 20 nM. Injection volume was 5 μL. The integration baseline (red) is shown for the raw data. The bottom figure is the Michaelis-Menten plot, fit with the single-injection enzyme kinetics model included in the PEAQ-ITC software. MicroCal PEAQ-ITC software determined that ΔHapp = -10.9 kcal/mol, kcat = 18.2 s-1 and KM = 2.8 μM. [Data from Malvern Panalytical].
Enzyme kinetics experiments can be performed with the addition of a competitive inhibitor in the ITC cell, using the multiple injection or continuous method. See Figure 4 for continuous assays of the hydrolysis of BAEE by trypsin in the absence or presence of the inhibitor benzamidine. KI was determined to be 15 μM with the PEAQ-ITC data analysis software.
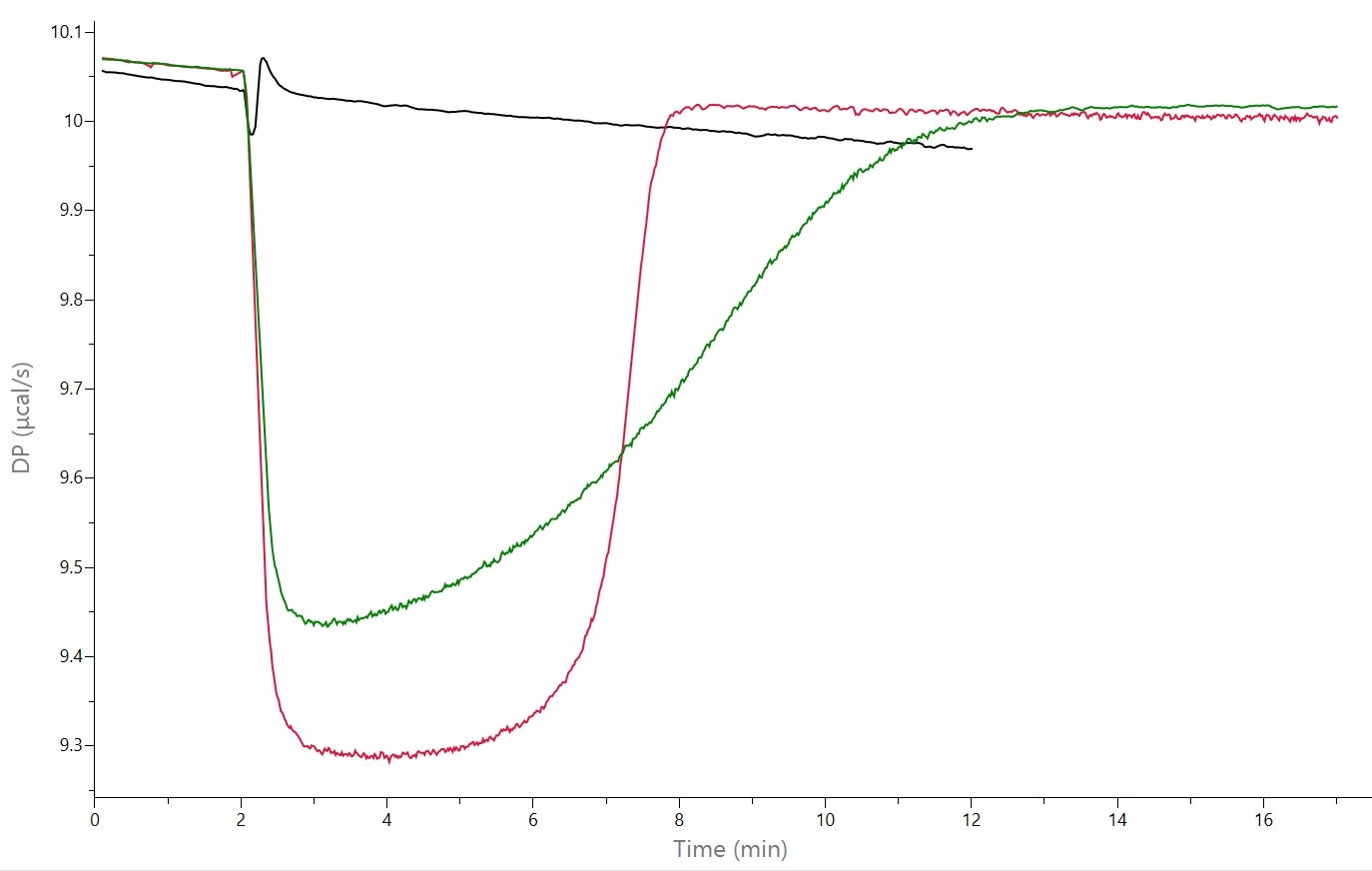
Figure 4: Raw ITC data (Top) and fit data (bottom) for the hydrolysis of BAEE with trypsin, collected on a MicroCal PEAQ-ITC. Black: control experiment, with 4 mM BAEE in the ITC syringe and buffer in the ITC cell; red: 4 mM BAEE in ITC syringe, and 20 nM trypsin in ITC cell (no inhibitor): green: 4 mM BAEE in ITC syringe, and 20 nM trypsin + 95 μM benzamidine (inhibitor) in ITC cell, Injection volume was 5 μL. [Data from Malvern Panalytical].
The efficient and accurate measurement of enzyme kinetics is crucial to technical advancement and optimization in life sciences research, drug discovery and development, and across a number of industries. ITC is a powerful and flexible technique for studying molecular interactions and can provide the information needed to generate a full Michaelis-Menten kinetic curve in less than 2 hours. High sensitivity means that sample requirements are low and allows experimentation with very dilute systems. Relative to traditional strategies based on chemical-reducing-end assays, ITC can be faster, less manually intensive and more informative.