
Physicochemical analysis solutions and expertise to facilitate drug product formulation development, aligned with bioavailability and processing requirements

Pharmaceutical formulation development links the discovery of a new drug substance to the successful development of a commercial drug product. Formulation development scientists must determine the most appropriate route to achieving effective drug delivery based on patient need, then optimize the formulation’s characteristics based on a knowledge of the drug product’s bioavailability and processing requirements.
This is a difficult challenge. Only 10% of new drug products in preclinical formulation development successfully reach the market. With the costs of pharmaceutical development rising, and pressure to release drug pipeline value also increasing, innovator drug development companies are applying significant effort to determining how to speed effective formulation selection. Developing a complete understanding of the form and structure of the drug substance and drug product is seen as a key enabler.
Similar challenges exist for generic pharmaceutical companies, where successful development of complex drug formulations remains difficult. There is a significant untapped market for new complex generics. Guided by global regulators, it is now recognized that detailed physicochemical understanding of the microstructure of complex dosage forms can enable successful product development.
Malvern Panalytical’s physicochemical techniques are routinely applied to address these formulation challenges. Our solutions are used to characterize physicochemical properties and better understand the drug substance and drug product form, to aid excipient selection and understand excipient function within formulations, and to assess how storage or processing may affect drug product performance. Our expertise enables the right data to be delivered, providing insights which unlock formulation development success.





Development of a successful pharmaceutical formulation requires the combination of the active pharmaceutical ingredient (API) with inactive excipients. Excipients may be simple bulking agents, designed to aid control of the dose content uniformity. Increasingly, though, some excipients have a functional role in controlling drug release or ensuring the drug reaches the desired site of action. Here, compatibility between the selected excipient and the drug substance is critical in ensuring the correct dose is delivered within the required therapeutic window. Physicochemical analysis can aid excipient selection, enable the stability of the drug substance and drug product to be assessed, and also ensure the critical material attributes (CMAs) relating to formulation performance are identified as part of the design space definition applied for downstream manufacturing controls.
The Zetasizer Ultra can characterize the stability and quality of dispersions, emulsions and creams, reducing formulation time and speeding new products to market.
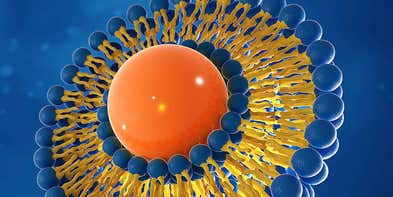
The increasingly complex requirements for achieving reproducible drug delivery are a common challenge for formulation development scientists. Many new active pharmaceutical ingredients (APIs) are poorly soluble, meaning that traditional oral solid dose delivery is no longer relevant. Formulation complexity is therefore increasing, either to enable increased bioavailability for oral administration, or to enable local delivery so that the drug concentration at the site of action meets therapeutic requirements.
Novel drug delivery systems, based on liposomes or other nanoparticle delivery systems, are being utilized more frequently to improve drug targeting. Malvern Panalytical’s range of complementary analysis techniques enables formulation developers to understand API and excipient formulation and stability. This aids the optimization of complex formulations, saving time in selecting an effective candidate formulation.
The challenges of developing complex formulations also extend to the development of generic drug products. Regulators around the world have recognized the impact of a lack of successful complex generic product introductions on healthcare costs. In response, they have released product-specific guidance which highlights the role of assessing physicochemical, or Q3, equivalence as part of the evaluation of the bioequivalence of a test generic product compared with a reference listed drug (RLD) product.
Application of an in vitro bioequivalence testing approach has the potential to significantly reduce the time to market for new generics by removing the need for clinical endpoint studies. Malvern Panalytical’s toolkit of physicochemical analysis techniques and expertise which enables assessment of the properties of both the drug and the drug product formulation has a critical role to play in enabling successful in vitro bioequivalence studies.

Development of a sucessful generic formulation starts with an understanding of the structure and performance of the reference listed drug (RLD) product. Here, as in in vitro bioequivalence assessments, physicochemical analysis has an important role to play in advancing the understanding of pharmaceutical formulation requirements. Proactive, quantative, structural and morphological characterization of the API and excipients present within the RLD product can prototype formulation optimization and significantly reduce development risks.
The benefits of this deformulation approach are not limited to generics companies. Similar methods are also applied in the development and manufacture of new drug products, providing insight to help pinpoint the root cause of changes in formulation performance during scale-up. It can also aid companies in understanding the impact of post-marketing changes to the manufacturing process or manufacturing location on the performance of a drug product.
The Morphologi 4-ID can be used to simplify and solve deformulation challenges and help establish in vitro bioequivalence. It can also be used to detect anomalies, contaminants and pinpoint process deviations during manufacturing.