Bio-Layer Interferometry (BLI) vs Surface Plasmon Resonance (SPR) vs Grating-Coupled Interferometry (GCI) comparison

Understanding the interaction between molecules – particularly the kinetics – can answer many questions, so it’s no surprise that it needs to be analyzed in many areas of research.
For instance, you may need to know how a molecule interacts with a receptor to understand how signaling occurs in an organism. Or you may want to understand whether, and how tightly, a drug binds to a compound of interest for drug discovery. The binding affinity can tell us this, but we can get more detail from measuring the binding kinetics.
The applications where binding kinetics are relevant, are as broad as the technologies, and several technologies can be used to study them. Here we compare three.
Note: for more information about measuring interactions, thermodynamics and more, please see our ITC technology page.
![[label free 2.png] label free 2.png](https://dam.malvernpanalytical.com/f38155b2-4fa2-4ed4-8366-ae6000effacd/label%20free%202_Original%20file.png)
Biolayer Interferometry (BLI) is an optical, surface-based, label-free technology. Unlike other biosensor technologies, BLI does not work with a microfluidic flow, but by immersion of sensor tips into the sample/buffer. Light reflected off the tip of an optical fiber exhibits a phase shift depending on the refractive index near the tip surface. The reflected light interferes with light reflected off an internal reference surface.
By using white light as a source, a spectral interference pattern is recorded, which comprises information about the refractive index near the tip surface. As biomolecules bind to the biolayer surface immersed in the experimental solution (such as a sample), the refractive index profile and thus the spectral pattern changes.
Surface plasmon resonance (SPR) is another optical, label-free analysis method – in fact, it was one of the first surface-based label-free technologies. SPR detects refractive index changes caused by molecular interactions within an evanescent field near a sensor surface.
In these sensors, a metal film on a glass support is illuminated with light of a specific wavelength. At a specific angle, depending on the refractive index close to the surface, so-called surface plasmons are excited. Since that energy is missing in the reflected light beam, a ‘dip’ in intensity is formed when projecting onto the sensor.
By determining the position of the dip in real-time, SPR measures changes in the refractive index on the metal surface. The solutions containing the analyte are injected using microchannels, and at least one reference flow cell is used for eliminating bulk effects.
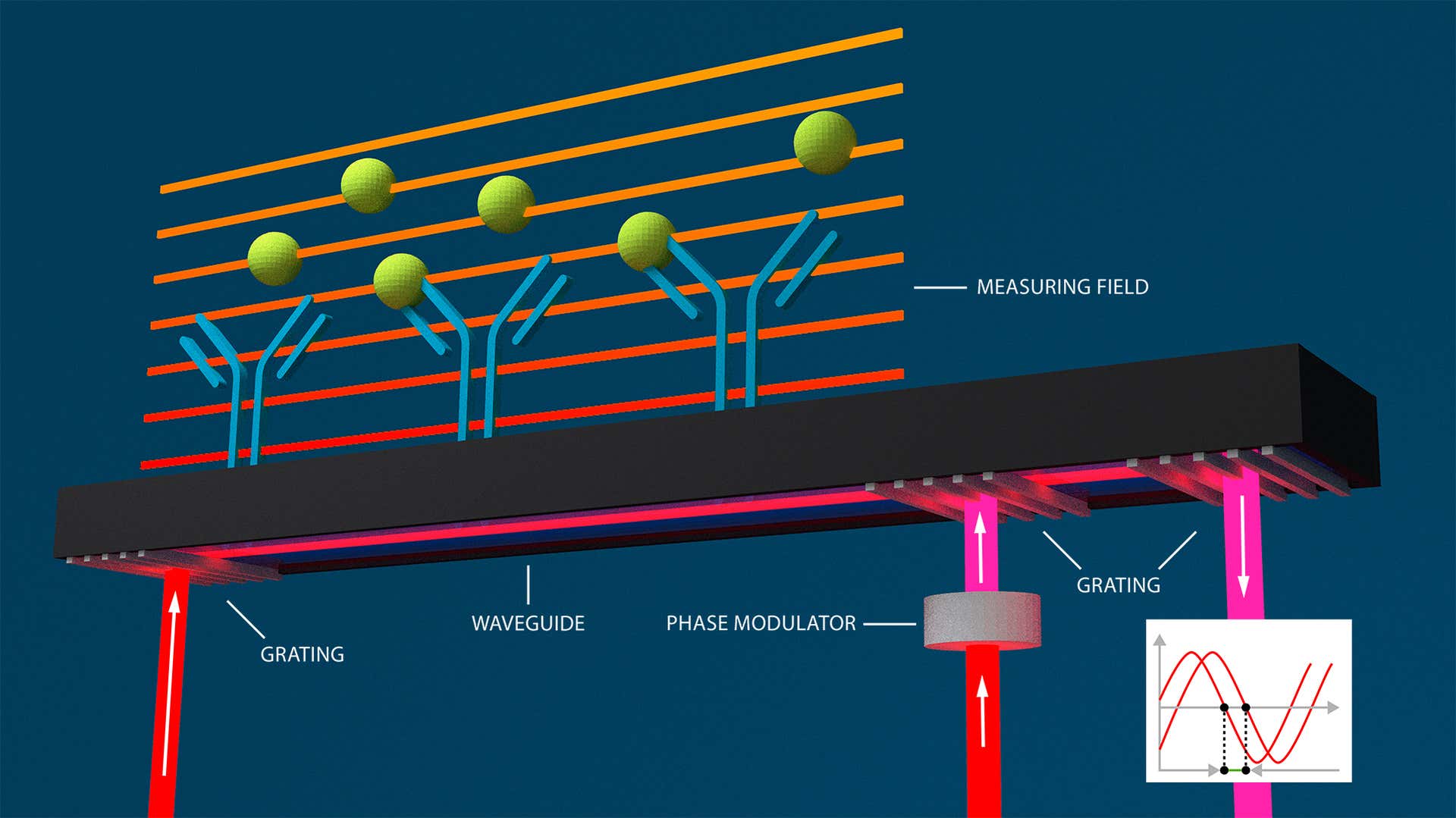
Based on waveguide interferometry – another optical label-free method – Grating-Coupled Interferometry (GCI) can monitor and characterize molecular interactions in real-time, determining kinetic rate parameters, affinity constants, and concentrations of analyte molecules interacting with an immobilized ligand.
In waveguide interferometry, changes in refractive index are measured within the evanescent field of a waveguide near a sensor surface. These changes cause the light phase to change too. The light travels throughout the waveguide, creating an evanescent wave that spans the entire length of the sensor surface. The phase changes are displayed interferometrically. Creoptix’s GCI technology takes the benefits of waveguide interferometry and eliminates its typical alignment issues:
![[GCI_CX_weblandscape.jpg] GCI_CX_weblandscape.jpg](https://dam.malvernpanalytical.com/b266ecf3-637f-41d5-a36a-ae29010df43c/GCI_CX_weblandscape_Original%20file.jpg)
The best biomolecular interaction technique will depend on the application and the user’s goals. Below, you can see how these three techniques compare for four key requirements: a broad application range, measurement of weakest binders, measurement of tightest binders, and low system maintenance.
| Grating-Coupled Interferometry (GCI) | Surface Plasmon Resonance (SPR) | Biolayer Interferometry (BLI) | |
|---|---|---|---|
| Broadest application range Suitable for a variety of molecules ranging from low to high molecular weights, purified or crude. | Yes Suitable for Fragments, Small Molecules, Peptides, Proteins, Viruses, Cell Culture Supernatants, Serums, Cell lysates | No Suitable for Small Molecules, Peptides (limited suitability for Fragments, Viruses, Cell Culture Supernatants, Serums, Cell lysates) | No Suitable for Cell Culture Supernatants, Serums, Cell lysates (limited suitability for Peptides, Proteins, Viruses) |
| Measure weakest binders Ability to measure kinetics with fast off-rates thanks to fast fluidics and high acquisition rates. | Yes Off-rates up to kd=10 s-1 | No Off-rates up to kd=1 s-1 | No Off-rates up to kd=0.1 s-1 |
| Measure tightest binders Ability to accurately measure kinetics even for tight binders and fast on-rates. | Yes Measurement under flow conditions | Yes Measurement under flow conditions | No Measurement under diffusion-limited conditions (no microfluidics) |
| Less downtime Little downtime due to service or unexpected repairs. | Yes No-clog microfluidics | No Traditional microfluidics | Yes No microfluidics |