The interaction of polymers with surfactants has been a subject of ongoing interest for a half century. Complexes of polyelectrolytes and oppositely charged micelles (PM complexes) can be important in cosmetics formulations [1], and may have environmental applications [2].
In the case of PM complexes, the driving force for association is electrostatic, and therefore highly dependent on the ionic strength (I) and the extent of charge on the polymer and the micelle. The micelle surface charge is easily manipulated by the ratio of ionic to non-ionic surfactant, or more fundamentally, the mole fraction (Y) of ionic surfactant, which is closely related to the micelle surface charge density. Complex formation is also influenced by the polymer molecular weight and concentration, and the polymer: surfactant stoichiometry.
We previously studied complexation of a model system [3], comprised of poly(dimethyldiallylammonium chloride) (PDADMAC), along with mixtures of Triton X-100 (TX100), and sodium dodecyl sulphate (SDS) in NaCl solutions with ionic strengths ranging from 0.05 to 1.5M. As reported in these earlier studies, dynamic light scattering is a powerful technique for characterizing these types of macromolecular complexes. In this report we investigate the influence of temperature on soluble complexes of PDADMAC with mixed micelles of SDS and TX-102, a non-ionic surfactant with a larger head group size than TX100. Preliminary studies indicate that the larger head group preserves the soluble complex state over a wider range of conditions. However, a characteristic feature of such systems is a lower critical solution temperature [4], and it is of particular interest to explore the properties of the system as this critical point is approached.
TX-102 from Sigma-Aldrich (St. Louis, MO) was used without further purification. Narrow molecular weight fractions of PDADMAC (141 kDa) was obtained from W. Jaeger, Fraunhofer Inst., Golm, Germany. 0.3 g/L PDADMAC was mixed with 20mM TX-102, both in 0.40 M NaCl, and titrated with 60.0mM SDS to bring the SDS mole fraction (Y) to 0.44. After filtration with 0.2µm Whatman filters, dynamic light scattering size measurements were collected with a Malvern Zetasizer system across a temperature range of 25 to 49°C.
This particular macromolecular complex system becomes biphasic at high temperature, with a turbidity discontinuity observable at a well-defined temperature (Tφ) of 47°C, and the data at somewhat lower temperatures were of particular interest in this study.
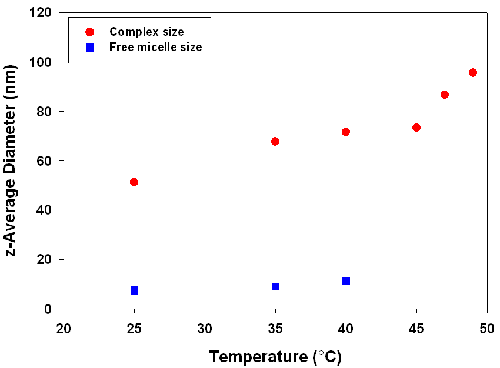
Figure 1 shows the measured hydrodynamic diameters of scattering species as a function of temperature. At all conditions, free micelles appear in equilibrium with complex, although when complexes are sufficiently large the signal from free micelles may be hard to detect. Uncomplexed polymer, if present, would not scatter sufficiently to be detected, although it is relevant to note that independent measurements of PDADMAC indicate a hydrodynamic diameter of 21nm [5].
|
As seen in Figure 1, the measured sizes of both the soluble complex and the free micelle grow with increasing temperature. The 50% increase in micelle size is not surprising, since TX-102 alone exhibits a cloud point of 91°C, suggesting substantial micelle growth with increasing temperature. Complexes also exhibit a similar change in size but this growth is non-monotonic and reaches a plateau at around 40°C. Most significant however, is the rapid growth of the complex from 45 to 47°C, previously established [4] as Tφ, the condition of incipient liquid-liquid phase separation or coacervate formation.
The species observed at 49°C, with a hydrodynamic diameter of circa 100nm, can be understood to arise from the aggregation of intrapolymer complexes, since the condition studied here (Y = 0.44) corresponds to a local maximum in turbidity. Such maxima are known to correspond to the formation of an electrically neutral complex subject to higher order aggregation. The results shown here suggest that soluble complex aggregation is a precursor to coacervation, in that the measured diameter of circa 100nm is 5 fold greater than the 21nm diameter of the free polyelectrolyte.
Polyelectrolyte-micelle binding is accompanied by a positive entropy change [5], and is therefore favored by increased temperature. The temperature increase facilitates the release of counter ions, a consequence of the electrostatic interactions between the polyelectrolyte and the micelle. Apparently, this effect also applies to the aggregation of intra-polyelectrolyte complexes, and to their higher-order aggregation up to the point of macroscopic phase separation.
[1] E.D. Goddard (1990) J. Soc. Cosmet. Chem. 41, 23.
[2] Y. Wang, J. Banziger, P.L. Dubin, G. Filippeli and N. Nuraje (2001) Environ. Sci. Technol. 35, 2608.
[3] Y. Wang, K. Kimura, Q.R.Huang, P.L. Dubin and W. Jaeger (1999) Macromolecules32, 7128.
[4] A. Kumar, P.L. Dubin, M.J. Hernon, Y. Li and W. Jaeger (2007) J. Phys. Chem. 111, 8468.
[5] L. Xia, P.L. Dubin, E. Kokufuta, H. Havel and B. Muhoberac (1999) Biopolymers50, 153.