The temperature-controlled humidity system for X-ray diffraction was applied to measure the influence of temperature and humidity on the crystal structure of trehalose.
Trehalose is a naturally occurring non-reducing disaccharide consisting of two glucose molecules. Understanding of the stability conditions and properties of the different phases is essential for the optimization of production processes and storage conditions for drugs.
Trehalose is a naturally occurring non-reducing disaccharide consisting of two glucose molecules. It plays an important as cryoprotectant in production of protenious drugs and it also prevents proteins in plant cells and other molecules from damaging upon freezing.
Understanding of the stability conditions and properties of the different phases is essential for the optimization of production processes and storage conditions for drugs. In the past X-ray diffraction in the vicinity of the stability limits of trehalose were difficult due to dew point limits of commercially available setups.
Thanks to the latest developments in temperature-humidity generation, it was possible for the first time to measure X-ray diffraction data at relative humidities up to 95 % and 70 % at 60 °C. The whole area between 25-80 °C and 5-95 % relative humidity was measured, not only by varying the humidity at constant temperature (as commonly done), but also by varying the temperature at constant humidity and by following the dew-point curve.
Features
Temperature controlled humidity systems offer simultaneous control of both humidity and temperature. The latest cryo- and humidity chamber (CHC Plus+) has been designed by Anton Paar GmbH to fit an Empyrean in a wider temperature - humidity range than it was possible before (see Fig 2.). Under dry gas or vacuum the experiments can be conducted at up to 400 °C and down to -180 °C with the additional liquid nitrogen equipment.
Application areas
X-ray diffraction measurements at defined environmental conditions are essential for lots of application areas:
• pharmaceuticals
• zeolites
• clays
• fine chemicals
• hydrating minerals
• hydrogen fuel cell research
In both research and industry, there is an increasing need to investigate the influence of temperature changes and different relative humidities on, for example, crystal structure, phase transitions or polymorphism of samples.
The impact on pharmaceutical research
At a high humidity level, in combination with exposure to elevated temperatures - for instance during storage or transport - an unexpected phase transition in pharmaceutical substances can occur. This can turn a powerful medicine into a useless or even dangerous substance. This is the first commercial humidity chamber with which you can conduct experiments that cover the compliance range of the stringent Japanese Pharmacopeia that requires a stability test at 70 °C.
X-ray diffraction measurements were performed on an Empyrean system equipped with a PIXcel1D and an Anton Paar CHC Plus+ setup.
With the new CHC Plus+ chamber it is possible to measure the humidity and temperature inside the chamber resulting in an optimal control of these parameters in the direct vicinity of the sample.
The maximum temperature of the CHC Plus+ setup is 60 ºC at a maximum relative humidity of 95 %. The maximum temperature is even 80 ºC for relative humidities up to 70 %. 2θ scans were recorded following different trajectories in the RH-T diagram:
a. varying RH while keeping T constant
b. varying T while keeping RH constant
c. following constant dew point curves
Starting conditions of all measurements were at room temperature and the lowest possible relative humidity (RH ≈ 5 % in dry N2).
Settling times were typically of the order of an hour. By scanning during the settle time the kinetics were monitored. Typical scan times were of the order of 15 minutes.
| Instrument | Empyrean |
|---|---|
| Radiation | Cu Kα at 45 kV, 40 mA |
| Programmable divergence slit
Anton Paar CHC Plus+ Programmable anti-scatter slit PIXcel1D | |
| Scan parameters | 6- 30 deg 2θ; stepsize 0.0167 deg; total scan time 30 minutes |
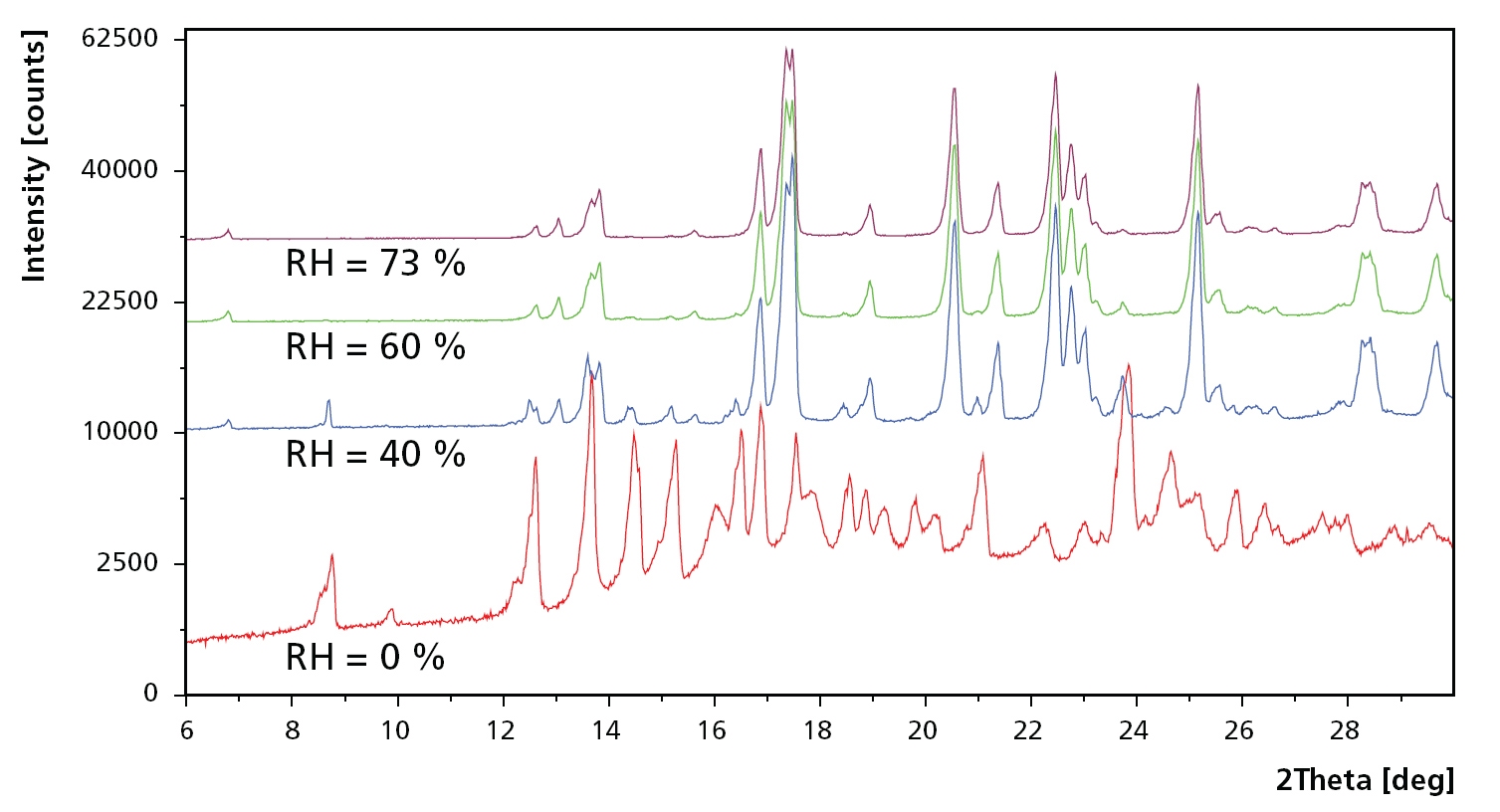
Figure 1. Example scans at 80 °C and relative humidity at 5, 40, 60 and 73 %RH. At 5 %RH, the sample consists of a mixture of trehalose in amorphous form (Ta), dehydrated form (Th) and the unstable anhydrate (Tα ). Ta vanishes when humidity is increased to 40 %RH, while Tα changes to the stable anhydrate Tβ when humidity is increased even further.
Trehalose
Commercially available crystalline trehalose dihydrated (C12H22O11·2H2O) with particle size between 10 μm and 100 μm was used to study the phase transformations. This form of trehalose is also known as Th and is used in this form in its typical applications such as cryoprotectants.
The structure of trehalose has extensively been studied in the literature using different techniques such as FTIR, Raman and XRPD, see [1] and the references therein. Apart from the dihydrated form (Th), two polymorphs of trehalose anhydrate (C12H22O11) exist: a stable form Tβ and an unstable one Tα. There exists also an amorphous form of trehalose (Ta).
The form in which trehalose occurs depends strongly on temperature and humidity. Furthermore, heating rate in combination with relative humidity and particle size are important factors for the formation of the different phases.
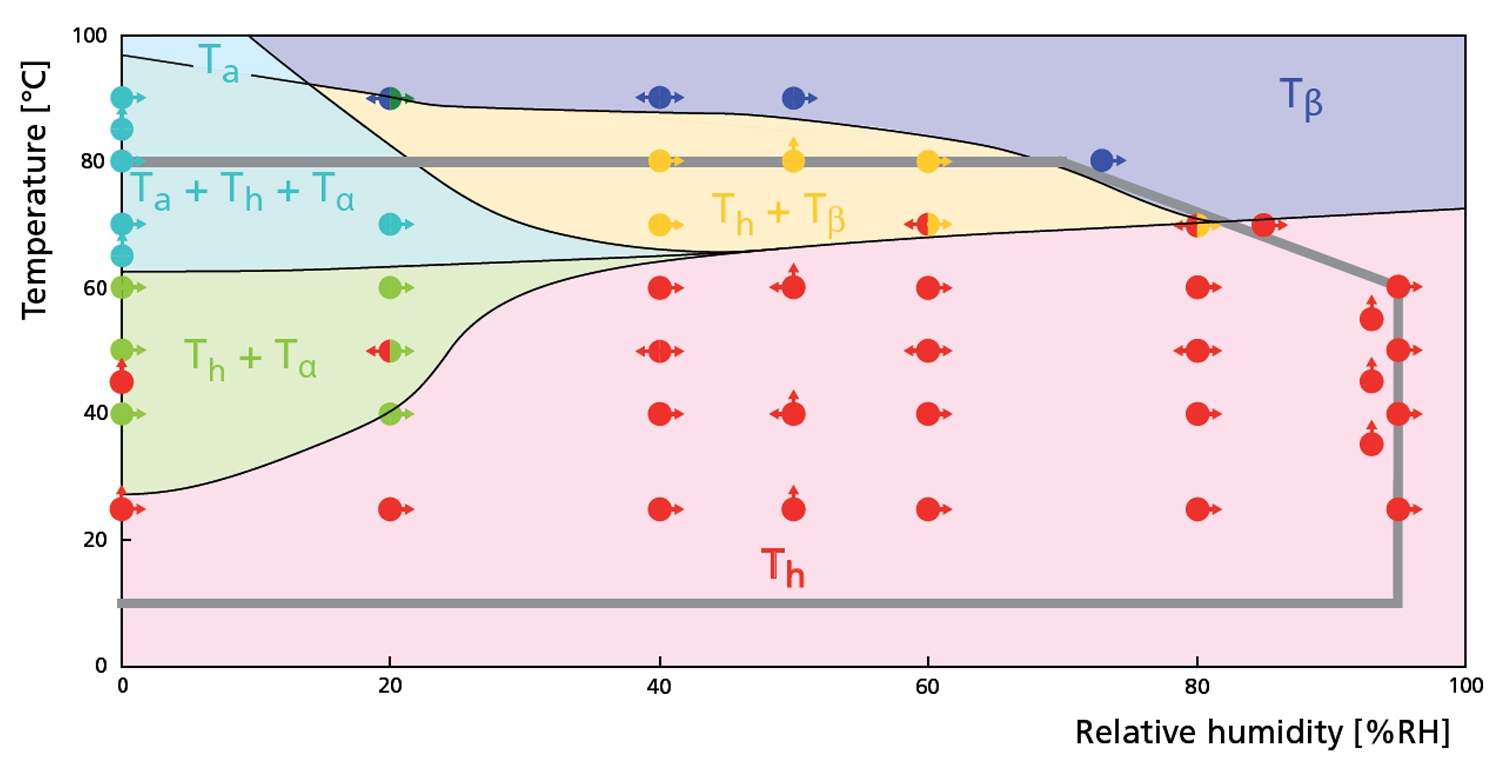
Figure 2. RH - T phase diagram derived from constant temperature and constant relative humidity experiments. The gray line indicates the maximum RH-T range.
Same RH-T ’phase‘ diagram now indicating two experiments where the dew point was kept constant (Tdew= 49 and 68 °C). The phases indicated in the diagram are indicated here as well. Note that once in form Tβ, trehalose does not return to form Th during cooling.
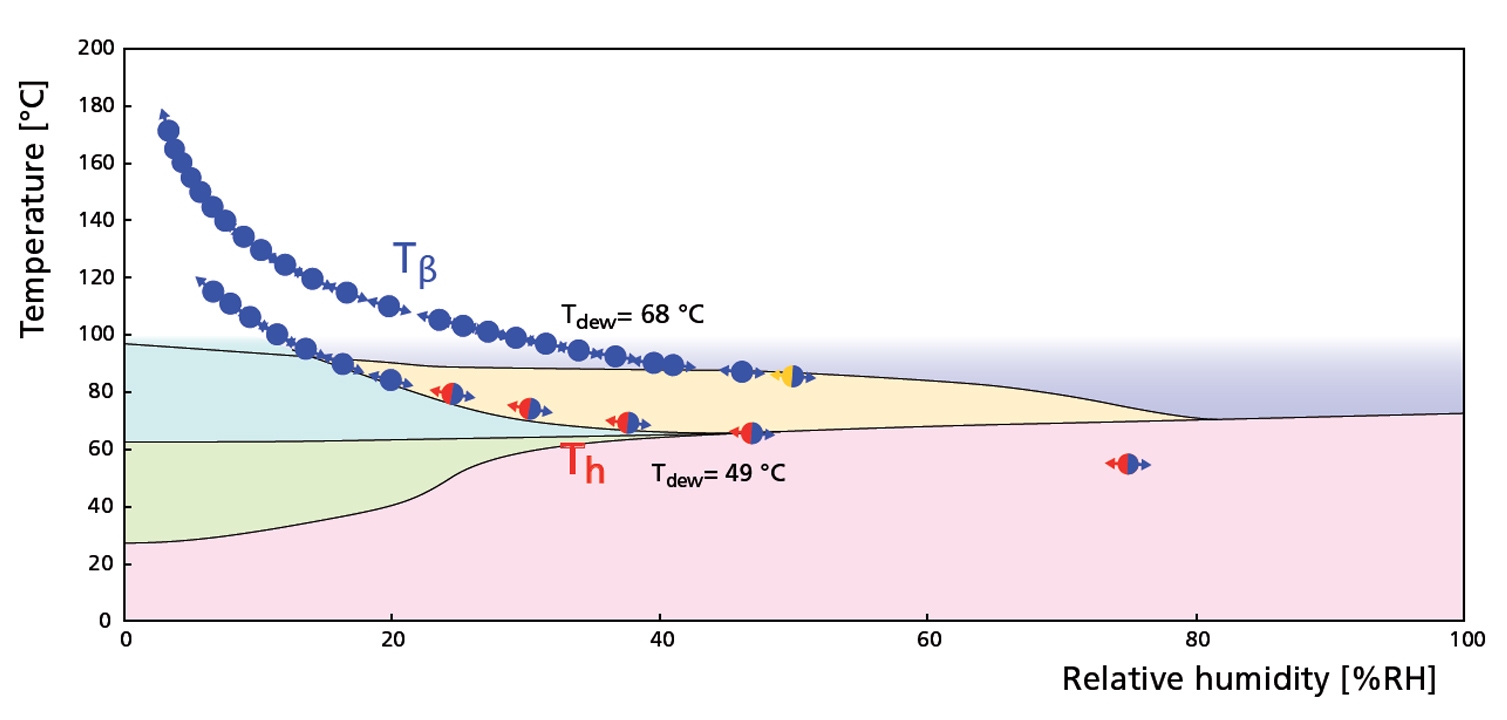
Figure 3. RH - T diagram derived from constant dew point experiments
Cluster analysis showing how form Th changes to form Tβ when increasing the temperature from 25 °C to 80 °C at 50 %RH.
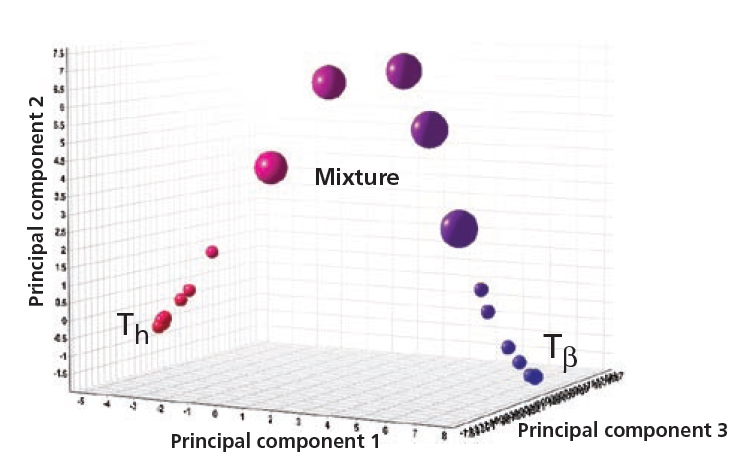
Figure 4. Anhydrate (Tβ ), dihydrate (Th) and their mixtures
2θ scans during heating and cooling at 20 %RH. For this experiment relatively short settling times of 15 minutes were used with temperature steps of 10 ºC. In this case the Tα form was not found and Th instantly changed to Tβ. After cooling trehalose did not return to Th, showing the stability of Tβ.
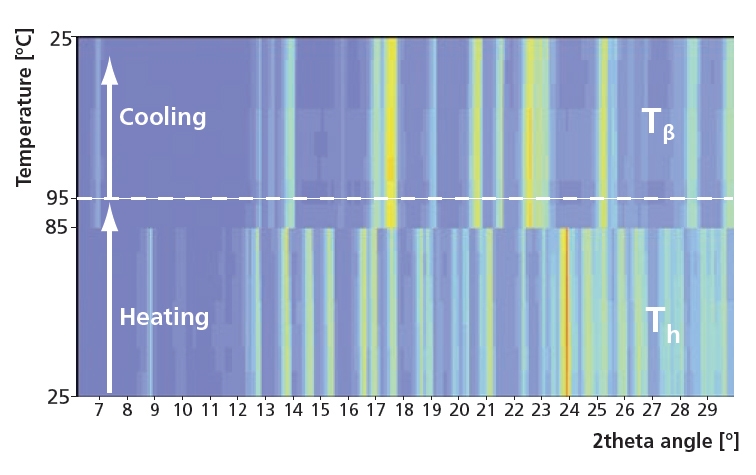
Figure 5. Instant phase change from Th to the stable form Tβ
With the Anton Paar CHC Plus+ it is possible to measure at exceptionally high relative humidity and temperatures which makes it possible to study a stable material like trehalose. It also allows measurements at constant relative humidity and varying temperature, without the risk for condensation.
Trehalose dihydrate (Th) shows to be very stable in a wide RH-T range. On the other hand, trehalose anhydrate, polymorph Tβ, is very stable at high temperatures. Once formed it seems that Tβ is also stable at lower temperatures.
We have not observed trehalose anhydrate, polymorph Tα in a pure form, only in a mixture with Th and together with the amorphous form Ta. It is possible that the settling time should be significantly longer to form pure Tα.
The strong dependence on heating rate and rate of humidity change is confirmed by very rapid heating at a constant relative humidity of 20 %rH where we observed an instant phase change to Tβ without an indication of a mixture with Tα.
Acknowledgements go to Christian Resch of Anton Paar.
The temperature-controlled humidity system for X-ray diffraction was applied to measure the influence of temperature and humidity on the crystal structure of trehalose.
Trehalose is a naturally occurring non-reducing disaccharide consisting of two glucose molecules. Understanding of the stability conditions and properties of the different phases is essential for the optimization of production processes and storage conditions for drugs. etc.
[1] A. Cesaro, O. De Giacomo, F. Sussich, Food Chemistry 106, 1318–1328 (2008)