In the development of injectable biotherapeutics, drug efficacy and patient experience are both very important parameters. In order to maximize activity and efficacy, biotherapeutics are usually delivered intravenously or subcutaneously in high concentrations, typically >100 mg/mL, due to their short half-life in plasma. Low injection volumes are required to minimize the invasiveness of the treatment for the patient. The parenteral administration of high concentration, low volume biotherapeutic formulations can be problematic due to significantly increased solution viscosities observed under these conditions.
The addition of small molecule excipients, such as arginine, dimethyl sulfoxide (DMSO) [1] and hydrophobic salts [2], has been shown to reduce the viscosity of highly concentrated protein solutions by inhibiting the formation of aggregate networks. The ability to screen formulations at an early stage in development for low viscosity attributes or against defined viscosity thresholds provides an attractive mechanism for the selection of biotherapeutic candidates with improved syringeability and injectability. This selection process can provide a shorter, more cost-effective route to bring a biotherapeutic to market.

|
The Viscosizer 200 is a benchtop system that performs automated viscosity measurements on very low volume samples (Figure 1). In this application note, we use the Viscosizer 200 to investigate the effect of an excipient on the viscosity of protein formulations.
Stock solutions of approximately 400 mg/mL Bovine Serum Albumin (BSA; Sigma Aldrich, Poole, UK) were prepared in two different buffers: (1) 30 mM histidine, pH 5.3 and (2) 30 mM histidine, 200 mM arginine.HCl, pH 5.3. From these stocks, a series of dilutions were prepared in the respective formulation buffers and the concentration of each new formulation determined by UV spectroscopy using the E1% at 280 nm for BSA (Nanodrop 200, Thermo Scientific, Wilmington, USA). The samples underwent no further treatment. Aliquots of 100 µL were transferred into small vials and placed into the autosampler carousel at 20oC. For viscosity measurements, samples were then loaded into an uncoated fused silica microcapillary at 1000 mbar and the progress of the sample front between the two detection windows was monitored at 214 nm. The capillary was washed with 30 mM histidine buffer and rinsed with pure water at 2000 mbar between measurements.
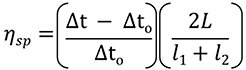
|
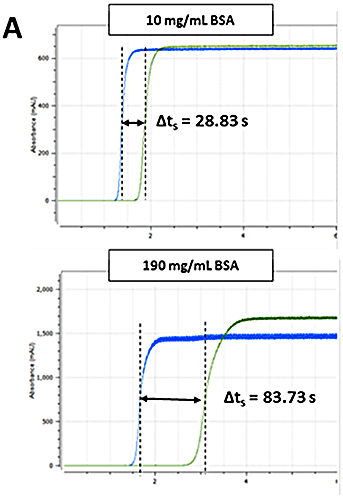
The Viscosizer 200 software (Malvern Instruments Ltd., UK) was used for all aspects of autosampler control, data acquisition and data processing. The ηsp was determined for each formulation using the relationship given in Equation 1. Frontal analyses of viscosity traces - shown in Figure 2 A - were used to obtain Δt and Δt0 for the sample and viscosity reference (pure water, 1.0021 cP), respectively. The remaining parameters - L, l1 and l2 - are known from the capillary dimensions.
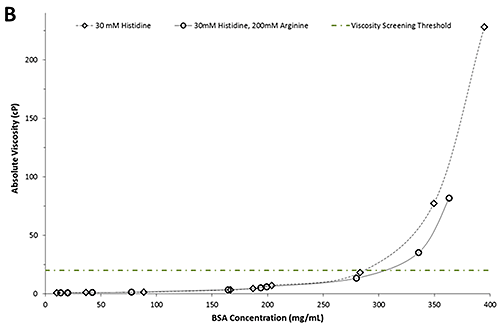
Figure 2B shows a plot of the absolute viscosity as a function of BSA concentration. In line with expectations, the absolute viscosities increase with increasing concentration for both formulations. The green dotted/dashed line represents a typical viscosity screening threshold for candidate selection.
|
|
A comparison of the absolute viscosities reveals no discernible differences between formulations of either buffer in concentrations below 250 mg/mL. However, in formulations with concentrations of more than 250 mg/mL, the BSA formulations in 30mM histidine have a notably greater viscosity than those containing arginine. For example, at 20 cP the BSA formulation containing arginine is approximately 30 mg/mL more concentrated than the BSA formulation in the histidine buffer alone. The apparent viscosity-lowering ability of arginine is consistent with studies conducted on monoclonal antibodies [1, 3]. Importantly, this information can be used to select formulation candidates based on low viscosity attributes. In the example above, four formulations fall above the viscosity screening threshold of 20 cP and consequently would be excluded from further development.
The incorporation of biological material in medicines presents an added layer of complexity to the process of drug development and manufacture. The production of high concentration, low viscosity biotherapeutic formulations is one of the key challenges faced by the pharmaceutical industry today. In this application note, we showed that the Viscosizer 200 could discern differences between the viscosities of different protein formulations and further demonstrated how this technology could be implemented as a tool in the screening of appropriate formulation candidates against a defined viscosity threshold. This would enable potentially problematic candidate molecules to be excluded at an early stage from future development, resulting in significant savings in time and investment.