The Zetasizer Advance series has the capability of measuring the size, particle concentration and zeta potential of colloidal dispersions using dynamic light scattering (DLS) and electrophoretic light scattering (ELS) [1-3]. These techniques are used extensively in lipid nanoparticle and liposome research for characterization purposes [4,5]. The number of peer reviewed papers highlighting the use of the Zetasizer Advance and Nano in these research areas exceed 50,000, confirming the versatility and usability of light scattering techniques.
Download this application note to learn how Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) can be used in the characterization of liposomes and lipid nanoparticles to understand their suitability for a range of different applications. You'll learn why size and zeta potential are key critical quality attributes (CQAs) in a range of formulations and how changes in these parameters can provide stability information.
The Zetasizer Advance series has the capability of measuring the size, particle concentration and zeta potential of colloidal dispersions using dynamic light scattering (DLS) and electrophoretic light scattering (ELS) [1-3]. These techniques are used extensively in lipid nanoparticle and liposome research for characterization purposes [4,5]. The number of peer reviewed papers highlighting the use of the Zetasizer Advance and Nano in these research areas exceed 50,000, confirming the versatility and usability of light scattering techniques.
Liposomes are vesicles in which an aqueous volume is entirely enclosed by a membrane composed of lipid molecules, usually phospholipid. They can be prepared so that they entrap materials both within their aqueous compartment (water-soluble materials) and within the membrane (oil-soluble materials) [7-9].
Lipid nanoparticles are currently being used in mRNA Covid-19 vaccines and consist of a neutral phospholipid, cholesterol, an ionizable cationic lipid and a PEGylated lipid. The PEGylated lipid is used to control the size of the lipid nanoparticles and acts as a steric stabilizer to prevent aggregation upon storage [10, 11].
The size and polydispersity of liposomes and lipid nanoparticles can be used as critical quality attributes of products. Stability can be studied by monitoring changes in particle size and polydispersity. For example, measurements taken over time will indicate the presence of any aggregation which might be present and help predict sample shelf life. Figure 1 shows the mean diameter (in nanometers) and polydispersity index of a liposomal formulation measured over time after their production. In this example, both the mean sizes and polydispersity index values remain stable over a 48 hour period confirming excellent sample stability. DLS is a technique particularly sensitive to the presence of aggregates due to its inherent intensity-weighting.
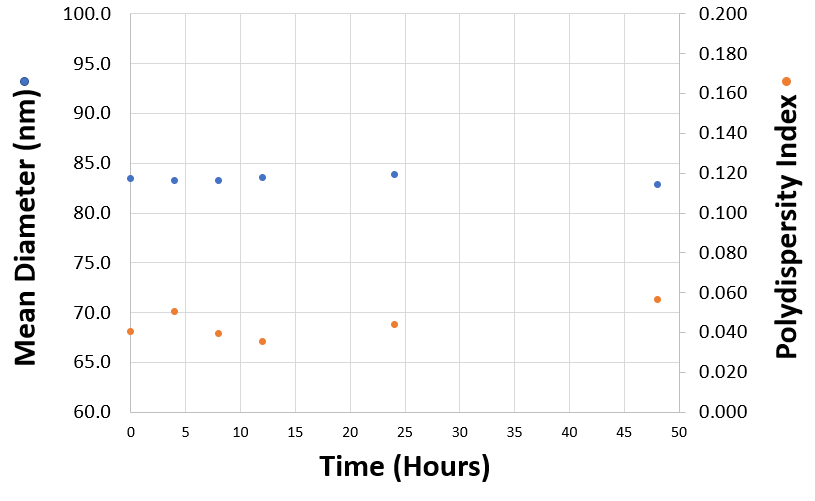
Figure 1: Mean diameters (in nanometres) and polydispersity index values obtained for a liposomal product measured as a function of time after their production.
Microfluidics is an increasingly popular method for rapid and scale-independent manufacture of liposomes and lipid nanoparticles. DLS can be used either offline or at line for optimizing microfluidic operating parameters and size monitoring can be used as a control parameter for scale up of the manufacturing process and batch release [5, 6]. Non-invasive backscatter (NIBS) optics present in the Zetasizer Advance and Nano product ranges allows for measurements of concentrated, turbid samples without the requirement of dilution [13].
Knowledge of the size and zeta potential of lipid nanoparticles or liposomes can help to predict their fate in vivo [7]. Any subsequent modification of the liposome surface can also be monitored by measurement of the size and zeta potential.
A major problem in the use of liposomes for the delivery of drugs by injection into the blood stream is the specific uptake of the liposomes by the reticuloendothelial system (RES). Longer circulation times can be achieved by coating the liposomes with a suitable polymer. The presence of hydrophilic polymers on the surface of the liposomes gives rise to a steric barrier which inhibits the adsorption of blood components which assist in the uptake of the liposomes by the RES [14].
One of the most successful hydrophilic polymers used to date for this purpose are polyethylene glycols (PEG) [15, 16]. Figure 2 shows a plot of the measured zeta potential of liposomes prepared in phosphate buffered saline (PBS) as a function of the concentration of PEG derivatized phospholipid (mole %). The zeta potential of ‘naked’ liposomes (with no PEG present on the liposome surface) is –43mV. The zeta potential starts to decrease with increasing concentration of PEG derivatized phospholipid and eventually reaches a plateau around –5mV.
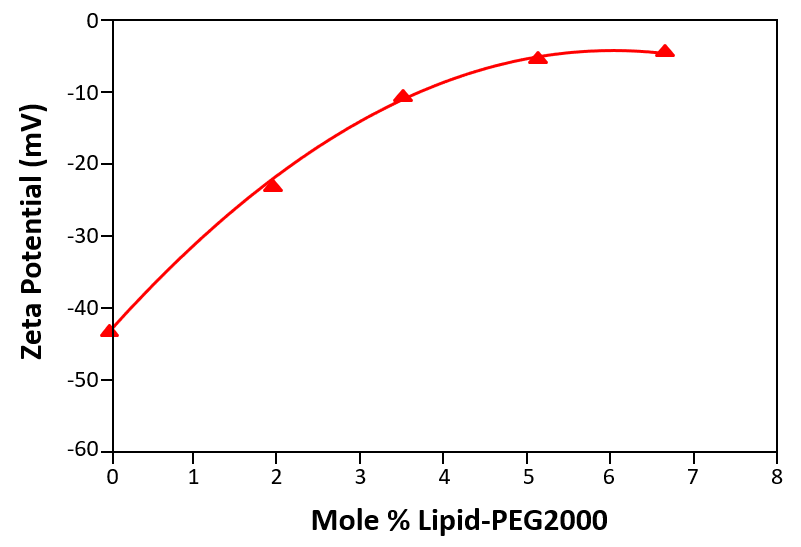
Figure 2: The zeta potential of liposomes prepared in phosphate buffered saline (PBS) as a function of the concentration of PEG derivatized phospholipid (mole %)
The decrease in the zeta potential as the surface of the liposome is covered can be explained in one of three ways, all of which are valid: (1) the charge of the liposome surface is “hidden” by the presence of the polymer layer, (2) the slipping plane is moved further away from the liposome surface hence reducing the zeta potential, (3) drag caused by the presence of the PEG chains on the liposome surface reducing the mobility of the liposomes (and hence the zeta potential).
The plateau region observed in figure 2 corresponds to the point at which no more PEG molecules can fit around the surface of the liposome. In the data shown, this point occurs at a concentration of 5 mole % of PEG derivatized phospholipid and demonstrates that zeta potential measurements can be used to determine the minimum concentration of polymer required to fully saturate the liposome surface.
In a recent study of soy lecithin liposomes, used for the delivery of antifungal and antiviral drugs, slower release rates were achieved when a coating of N-stearoyl chitosan was used. DLS and ELS measurements showed that the average size and zeta potential of the liposomes were significantly reduced by the coating layer [17].
Lipid nanoparticles and liposomes have been used as vectors for gene therapy applications [18, 19]. Cationic liposomes have been used to complex DNA plasmids, with the liposome: DNA ratio affecting optimal transfection into cells [20]. Size and zeta potential measurements allows for detailed characterization of such plasmid:liposome complexes.
Size and zeta potential measurements can be used optimise the ratio required for liposomes with various plasmids. Figure 3 shows the size (z-average diameters) and zeta potentials of cationic liposome/DNA plasmid complexes as a function of the molar ratio of liposome to plasmid.
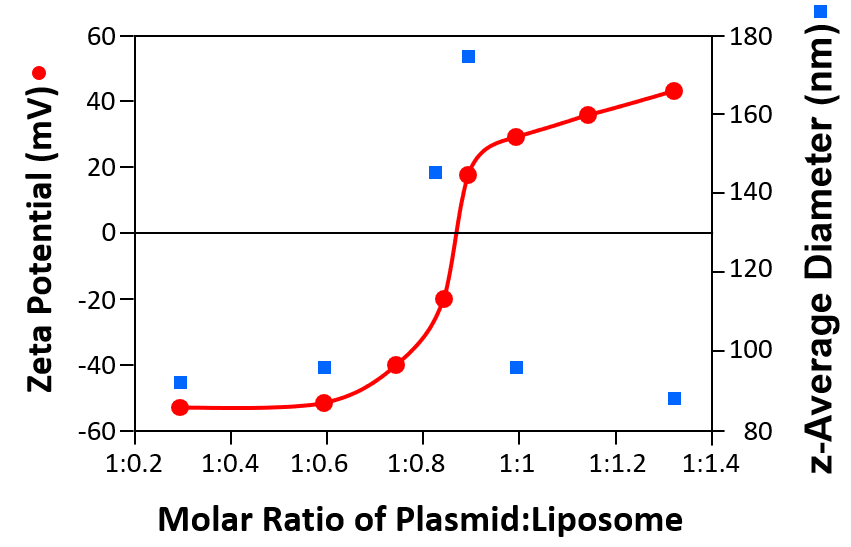
Figure 3: Sizes (z-average diameters) and zeta potentials of cationic liposomes/DNA plasmid complexes as a function of the molar ratio of plasmid to liposome
When the complex has either a high negative or positive zeta potential, the size is consistently around 90nm. However, when the zeta potential approaches the iso-electric point (the point of zero zeta potential), the size increases indicating aggregation of the complex due to reduced electrostatic repulsion. Zeta potential measurements in conjunction with size determination can be used to develop efficient formulations for transfection studies in vitro and in vivo.
The examples discussed above show how DLS and ELS can be used in the characterization of liposomes and lipid nanoparticles to understand their suitability for a range of different applications. Size and zeta potential are key critical quality attributes in a range of formulations and changes in these parameters can provide stability information.
[1] Malvern Panalytical Dynamic Light Scattering: An Introduction in 30 Minutes (https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/TN101104DynamicLightScatteringIntroduction.html).
[2] Malvern Panalytical Zeta Potential: An Introduction in 30 Minutes (https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/TN101104ZetaPotentialIntroduction.html).
[3] J. Austin, C. Minelli, D Hamilton, M Wywijas and H Jankevics Jones. Nanoparticle Number Concentration Measurements by Multi-Angle Dynamic Light Scattering, J. Nanopart. Res. 2020, 22, 108-123.
[4] Y. Fan, M. Marioli and K. Zhang. Analytical Characterization of Liposomes and Other Lipid Nanoparticles for Drug Delivery (2021) J. Pharma and Bio. Anal. 192, 113642.
[5] N. Forbes, M.T. Hussain, M.L. Briuglia, D.Y. Edwards, J.H.T. Horst, N. Szita and Y. Perrie. Rapid and Scale-Independent Microfluidic Manufacture of Liposomes Entrapping Protein Incorporating In-Line Purification and At-Line Size Monitoring Int. J. Pharmaceutics, 556, 68-81.
[6] Polymun Scientific on Liposome Technolohy
[7] T.M. Allen and P.R. Cullis. Liposomal Drug Delivery Systems: From Concept to Clinical Applications (2013) Advanced Drug Delivery Reviews 65, 36–48.
[8] M. Alavi, N. Karimi and M. Safaei. Application of Various Types of Liposomes in Drug Delivery Systems (2017) Adv. Pharm. Bull. 7, 3-9.
[9] D. Sharma, A.A.E. Ali and L.R. Trivedi. An Updated Review on Liposomes as Drug Delivery Systems (2018) PharmaTutor 6, 50-61.
[10] C. Wan, T.M. Allen and P.R. Cullis. Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv Transl Res. 2014 Feb;4(1):74-83. doi: 10.1007/s13346-013-0161-z.
[11] T.T.H. Thi, E.J. A. Suys, J.S. Lee, D.H. Nguyen, K.D. Park and N.P. Truong. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines (2021) Vaccines 9, 359- 388.
[12] P. Ghasemiyeh and S. Mohammadi-Samani. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages (2018) Res. Pharm. Sci. 13, 288-303.
[13] M. Kaszuba, M.T. Connah, F.K. McNeil-Watson and U. Nobbmann. Resolving Concentrated Particle Size Mixtures Using Dynamic Light Scattering (2007) Part. Part. Syst. Charact. 24, 1-4.
[14] T.M. Allen and C. Hansen. Pharmacokinetics of Stealth Versus Conventional Liposomes: Effect of Dose (1991) Biochim. Biophys. Acta 1068, 133-141.
[15] M.C. Woodle and D.D. Lasic. Sterically Stabilised Liposomes (1992) Biochim. Biophys. Acta 1113, 171-199.
[16] T.M. Allen, C. Hansen, F. Martin, C. Redemann and A. Yau-Young. Liposomes Containing Synthetic Lipid Derivatives of Poly(ethylene glycol) Show Prolonged Circulation Half-Lives in vivo (1991) Biochim. Biophys. Acta 1066, 29-36.
[17] E. Suk, V.R., Marlina, A., Hussain, Z. et al. N-Stearoyl Chitosan as a Coating Material for Liposomes Encapsulating Itraconazole (2021) Arab J Sci Eng. https://doi.org/10.1007/s13369-020-05327-3.
[18] D.D. Lasic and N.S. Templeton. Liposomes in Gene Therapy (1996) Adv. Drug Delivery Reviews 20, 221-266.
[19] R.K. Fisher, M.S. S.I. Mattern-Schain, M.D. Best, S.S. Kirkpatrick, M.B. Freeman, O.H. Grandas and D.J.H. Mountain. Improving the Efficacy of Liposome-Mediated Vascular Gene Therapy via Lipid Surface Modifications (2017) J. Surgical Research 219, 136-144.
[20] S. Simões, A. Filipe, H. Faneca, M. Mano, N. Penacho, N. Düzgünes & M. Pedroso de Lima. Cationic Liposomes for Gene Therapy (2005) Expert Opin Drug Deliv. 2, 237-254.