This white paper addresses some of the latest work reported in the literature in which Nanoparticle Tracking Analysis (NTA) has been proposed, used and assessed in the study of nanoparticle-based drug delivery and targeting
Nanoparticle Tracking Analysis (NTA) represents a rapid and information-rich multi-parameter nanoparticle characterization technique allowing the user to obtain number frequency particle size distributions of polydisperse nanoparticulate systems. It has resulted in its rapid adoption as an interesting new technique in a wide range of sectors within the pharmaceutical sciences. This white paper addresses some of the latest work reported in the literature in which NTA has been proposed, used and assessed in the study of nanoparticle-based drug delivery and targeting.
NTA utilizes the properties of both light scattering and Brownian motion in order to obtain the particle size distribution of samples in liquid suspension. A laser beam is passed through the sample chamber, and the particles in suspension in the path of this beam scatter light in such a manner that they can easily be visualized via a 20x magnification microscope onto which is mounted a camera. The camera, which operates at approximately 30 frames per second (fps), captures a video file of the particles moving under Brownian motion within the field of view of approximately 100 μm x 80 μm x 10 μm (Figure 1).
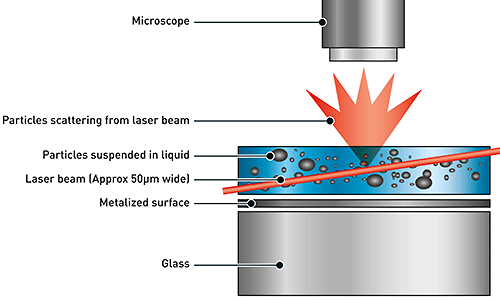
|
The movement of the particles is captured on a frame-by-frame basis. The proprietary NTA software simultaneously identifies and tracks the center of each of the observed particles, and determines the average distance moved by each particle in the x and y planes. This value allows the particle diffusion coefficient (Dt) to be determined from which, if the sample temperature T and solvent viscosity η are known, the sphere-equivalent hydrodynamic diameter, d, of the particles can be identified using the Stokes-Einstein equation (Equation 1).
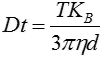
| Equation 1 |
where KB is Boltzmann’s constant.
NTA is not an ensemble technique interrogating a very large number of particles, but rather each particle is sized individually, irrespective of the others. An example of the size distribution profile generated by NTA is shown in Figure 2.
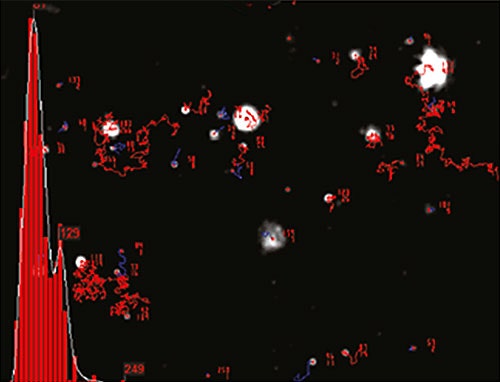
|
In addition, the particles’ movement is measured within a fixed field of view (approximately 100 μm by 80 μm) illuminated by a beam approximately 10 μm in depth. These figures allow a scattering volume of the sample to be estimated; by measuring concentration of the particles within this field of view and extrapolating to a larger volume it is possible to achieve a concentration estimation in terms of particles per mL for any given size class or an overall total.
It is well established that the use of nanotechnology in medicine and more specifically drug delivery is spreading rapidly. Driven by the diminishing rate of discovery of new biologically active compounds that can be exploited therapeutically to treat disease and with fewer new drugs entering the market every year, interest in the use of nanoparticle’s versatile and multifunctional structures for the delivery of existing drugs has grown rapidly. Nanoparticles offer better pharmacokinetic properties, controlled and sustained release, and targeting of specific cells, tissues or organs (e.g. in new ways in which to cross the blood-brain barrier). All these features can improve the efficacy of existing drugs (Malam et al., 2011). Nanoparticles in this context have been defined as colloidal systems of sub-micron size that can be constructed from a large variety of materials in a large variety of compositions. Commonly defined nanoparticle vectors include: liposomes, micelles, dendrimers, solid lipid nanoparticles, metallic nanoparticles, semiconductor nanoparticles and polymeric nanoparticles. Therefore, nanoparticles have been extensively employed to deliver drugs, genes, vaccines and diagnostics into specific cells/tissues (Ram et al., 2011).
However, while such nanoparticles are being increasingly used to reduce toxicity and side effects of drugs, it has been recognized that carrier systems themselves may impose risks to the patient. The kind of hazards that are introduced by using nanoparticles for drug delivery are beyond that posed by conventional hazards imposed by chemicals in classical delivery matrices. A multitude of substances are currently under investigation for the preparation of nanoparticles for drug delivery, varying from biological substances like albumin, gelatin and phospholipids for liposomes to substances of a more chemical nature like various polymers and solid metal containing nanoparticles. It has been previously recognized that the potential interaction with tissues and cells, and the potential toxicity, greatly depends on the actual composition of the nanoparticle formulation (De Jong and Borm 2008, Moquin and Winnik 2012).
Given the above, it is not surprising that the characterization of nanoparticles intended for drug delivery has been the subject of a recent review (McNeil, 2011a) in which the benefits of nanotechnology have been described but with warnings concerning the fact that the physical nature of the nanoparticles can interfere with conventional and standardized biocompatibility and immunotoxicity testing protocols. In his further comprehensive review of the subject, McNeil (2011b) has also described many assays to determine physical and chemical properties of nanoparticles including batch-mode dynamic light scattering (DLS), MALDI-TOF, zeta potential measurement, AFM, TEM and SEM X-Ray microanalysis of nanoparticles present in tissue or cultured cell thin sections. Nanoparticle Tracking Analysis, being a recently developed technique, was not considered in this review but is, however, gaining use in the characterization of nanoparticulate suspensions being developed for drug delivery usage, as is described below. An understanding of the dispersion of a distribution of nanoparticle sizes prior to their introduction to cellular systems for cytotoxilogical testing is crucial and NTA has proved useful in this regard compared to other nanoparticle characterization techniques such as DLS (Kendall et al., 2010).
Following early work using NTA for the study of sodium caproate mediated promotion of oral drug absorption (Maher et al., 2009), more recent work has used NTA to study holonium (Bult et al., 2010)
Moddaresi et al. (2010) used NTA to show that semi-solid gel hyaluronic acid matrices used for topical application of drug delivery nanovesicles (tocopheryl acetate (TA) lipid nanoparticles) did, as expected, inhibit their mobility but deliberate manipulation of the particle mobility in the gel by varying the concentration of HA had little effect on TA delivery showing that drug release from the lipid nanoparticles was the rate limiting step in the delivery process and not the nanoparticle–vehicle–skin interaction. Bhuiyan (2010) showed that localized drug release from thermosensitive liposomes could be induced by hypothermia using NTA to characterize his liposome preparations.
More recently, Sunshine et al. (2012), in developing safe and effective delivery system based on poly(beta-amino ester)s (PBAEs) which show great potential as gene delivery reagents because they are easily synthesized and transfect a wide variety of cell types with high efficacy in vitro, have used NTA to determine particle size just prior to subretinal injection. The successful transfection of the RPE in vivo suggested that these nanoparticles could be used to study a number of genetic diseases in the laboratory with the potential to treat debilitating eye diseases.
Shirali et al. (2011) used NTA in the development of a poly(lactic-co-glycolic acid) (PLGA) nanoparticle formulation. PLGA nanoparticles are among the most studied polymer nanoformulations for several drugs against different kinds of malignant diseases, thanks to their in vivo stability and tumor localization exploiting the well-documented “enhanced permeation and retention” effect. Similarly, in treating the endemic disease Paracoccidioidomycosis, through a new formulation comprising the sustained release of encapsulated itraconazole in nanostructured PLGA, NTA was used to establish an average size of 174 nm which showed that the encapsulated delivery system exhibited improved performance and reduced cytotoxic effects (Cunha-Azevedo, 2011). PLGA nanoparticles loaded with curcumin have been shown to induce G2/M block in breast cancer cells. Using NTA to show full precipitation of the nanoparticle preparation, the PLGA nanoparticles proved to be completely safe, suggesting a potential utilization of this nanocomplex to improve the intrinsically poor bioavailability of curcumin for the treatment of severe malignant breast cancer (Verderio et al., 2013).
Other examples of the importance of sizing and enumerating nanoparticulate drug delivery systems by NTA have been reported (Hsu et al. 2010, Park et al. 2010, Tagalakis et al. 2010).
The successful transport of molecules across the cell membrane is a key point in biology and medicine. In most cases, molecules alone cannot penetrate the cell membrane, therefore an efficient carrier is needed. Sokolova et al. (2012 a and b) have investigated calcium phosphate nanoparticles (diameter: 100–250 nm, depending on the functionalization) as versatile carrier for small and large molecules across cell membranes using a number of techniques including NTA, DLS and EM.
In studying lipid exchange between membranes and the effects of membrane surface charge, composition, and curvature, Zhu et al. (2012) employed a quartz crystal microbalance with dissipation monitor method and showed that vesicle adsorption rate, membrane lateral pressure gradient, and lipid lateral diffusion coefficient are critical in deciding the lipid exchange kinetics between membranes and that NTA-determined vesicle size was inversely proportional to membrane contact area which directly affected the intermembrane lipid exchange rate.
The hemocompatibility of poly(beta-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan as drug delivery systems has been studied by Bender et al. (2012) and NTA was also used to follow size changes in nanocapsules for intestinal delivery and enhanced oral bioavailability of tacrolimus, a P-gp substrate (Nyska and Benita 2009), as were other several other nanocarriers (Nasser et al., 2009; Debotton et al., 2010).
Most recently, Abdel-Hafez et al. (2013) have utilized statistical designs and mathematical modelling to address questions about the different variables that influence the production of nanoparticles using the ionic gelation method between the biopolymer chitosan and tripolyphosphate ion. Nanoparticles were produced with diameters ranging from 52.21 nm to 400.30 nm, particle polydispersity from 0.06 to 0.40 and suitable morphology. NTA was performed to visualize the prepared particles and to ensure the absence of aggregates.
Using DLS and NTA to confirm formulation unimodal size distribution (with polydispersity value <0.1 from DLS) at the nanoemulsion as well as multi-unit pellet system (MUPS) stage Sangwai et al. (2012) reported a nanoemulsified poorly water-soluble anti-obesity drug Orlistat-embedded MUPS with improved dissolution and pancreatic lipase inhibition.
In a study to develop curcumin-loaded lipid-core nanocapsules (C-LNC) in an attempt to improve the antiglioma activity of this polyphenol, visualization of the C-LNC was carried out by NTA (Zanotto-Filho et al., 2012), the data obtained suggesting that the nanoencapsulation of curcumin in LNC is an important strategy to improve its pharmacological efficacy in the treatment of gliomas.
Cunha-Azevedo also developed and tested an anti-fungal formulation of PLGA nanoparticles designed to release the active agent itraconazole in which size was an important feature and analyzed by NTA as an average of 174 nm (Cunha-Azevedo 2011).
For the development of novel thiolated dendrimers for mucoadhesive drug delivery, Yandrapu et al. (2012) used NTA to characterize the dendrimer conjugates showing they exhibited sustained release of acyclovir and higher mucoadhesion.
Similarly, Kumru et al. (2012) have studied the compatibility, physical stability and characterization of an IgG4 monoclonal antibody after dilution into different intravenous administration bags using a combination of SE-HPLC, NTA, microflow-digital imaging (MFI), and turbidity measurements to follow the formation of soluble aggregates and particulates. He noted, however, that NTA quantification results were interfered with by the presence of polysorbate 20.
In the formulation design and characterization of different nanoparticle drug delivery systems, knowledge of the size, size distribution and number concentration remains central to understanding the behavior of the system. NTA has been shown to be very useful in this regard. Katzer et al. (2013) developed a castor oil and mineral oil nanoemulsion as a promising ocular drug delivery system. The formulations were developed by spontaneous emulsification and NTA used to show a mean particle size of 234 nm. Other ocular drug delivery system has been investigated using hyaluronic acid-based nanocomposite hydrogels, HA being a natural component of eye tissue with a significant role in wound healing. The size distribution of liposomes slowly released from the cross linked substrate was determined by NTA which supported the idea that these nanocomposite hydrogels, with controlled degradation properties and sustained release, could serve as potential drug delivery systems for many ocular diseases (Widjaja et al., 2013).
NTA was used to determine the particle size distribution of hollow magnetic Fe3O4C nanoparticles as drug carriers with high drug loading capability, pH-control drug release and MRI properties (Cheng et al., 2012) and Morch et al. (2013) have developed a novel nanoparticle-microbubble platform in which NTA was the only technique suitable for its characterization.
Nanocapsule size of ~80 to ~100 nm was established by NTA in the work carried out by Piotrowsk et al. (2013) on the development of emulsion-core and polyelectrolyte-coated nanocapsules, designed as a water-insoluble neuroprotective drug delivery system. The results showed that nanoencapsulated forms of MDL 28170 were biocompatible and protected SH-SY5Y cells against the H2O2 (0.5 mM/24 h)-induced damage in 20–40 times lower concentrations than those of the same drug added directly to the culture medium.
The field of nanoscale drug carriers to enhance effective oral drug delivery has recently been reviewed by Reis et al. (2013) and Howard and D Peer (2013) concluded that “Nanoparticle Tracking Analysis based on video capture of the trajectory of individual particles provides high-resolution particle size discrimination in polydispersed samples and is gaining popularity in their assessment of different techniques for standardizing nanoparticle-based drug delivery systems, including DLS, SEM and TEM though high-resolution cryo-TEM with environmental-SEM techniques can provide measurements in more natural states”. Another overview of advanced technologies potentially applicable in personalized treatment included discussion of techniques based on the measurement of particle’s Brownian motion (DLS and NTA) and on centrifugal sedimentation (Figueiredo, 2013). The overview was aimed at users who are not very acquainted with particle sizing issues but need to select the most adequate method to characterize their suspensions acknowledging that the techniques are quite different in their measuring principles and that may lead to rather different results, especially if the particles under analysis are far from spherical and exhibit broad size distributions.
As structures capable of crossing biological barriers, such as the membrane linings of various body tissues and the skin, Mbah et al. (2013) have discussed the importance of various vesicular carriers, namely liposomes, niosomes, transfersomes and ethosomes in drug delivery with greater emphasis on ethosomes. They concluded that vesicular carriers offer controlled and sustained drug release, improved permeability and protection of the encapsulated bioactives and that ethosomes in particular offer more efficient and enhanced bioavailability better than the older dosage forms owing to the high ethanol content. NTA was cited as a suitable technique for particle size analysis which did not suffer from the intensity weighting exhibited by more traditional methods such as DLS.
Drug release and liposome destruction were determined by photoinduced quenching and NTA respectively in a recent study by Garrier et al. (2013) in which they addressed the factors affecting the selectivity of nanoparticle-based photoinduced damage in free and xenografted chorioallantoïc membrane models.
Mund (2013), in his study of titania nanoparticles for the intracellular delivery of paclitaxel in breast cancers, showed that NTA results determined more than 50% of particles aggregate in <100 nm dimension. He suggested that a concrete understanding of the particle size and concentration dependent dimension is able to predict drug loading and encapsulation efficiency and hence helpful in determining the effectiveness of drug-carrier conjugate in site dependent action and that therefore, simultaneous characterization through TEM and NTA is significantly valuable in drug delivery application.
Mendes et al. (2013) have recently described multi-compartimental nanoparticles for co-encapsulation and multimodal drug (paclitaxel and genistein), delivery to tumor cells and neovasculature, in which NTA was used to help demonstrate that entrapment efficiency for both drugs in the nanoparticles was approximately 98%. Average particle diameter was 150 nm with a monomodal distribution. In vitro assays showed distinct temporal drug release profiles for each drug and that nanoparticles containing paclitaxel and genistein with a temporal pattern of drug release indicated that the combined effect of cytotoxic and antiangiogenic drugs present in the formulation contributed to the overall enhanced antitumor activity of the nanomedicine.
Finally, NTA has been cited in numerous recent patent applications in the field of nanoscale drug delivery development (Bloembergen et al., 2013; Chen and Walsh, 2013 and Haag et al., 2013).
The targeting of drug delivery nanoparticles to specific sites frequently uses the addition of molecular structures with an affinity for specific cell surface biomarkers which allows the drug-containing nanoparticle to be accumulated by the target cell types presenting such biomarkers.
The addition of such capture molecules (frequently antibodies) to the surface of the drug delivery nanoparticle structure can, however, be problematic; retention of activity, sufficient loading and minimization of aggregation being necessary for optimum performance. Similarly, addition of other biochemical species designed to stabilize the functional structures added to the nanoparticles or which act to reduce the immunogenicity of the nanoparticle may result in similar deleterious effects. NTA is capable of detecting small changes in hydrodynamic diameter following the addition of layers of macromolecules to nanoparticles and can both detect and enumerate any aggregates which may form during such modifications.
Accordingly, NTA has been used in a number of such studies including the effect of conjugating polymer-alendronate-taxane complexes for targeting bone metastases (Miller et al., 2009). The same group used NTA to show that successful conjugation for the targeting of angiogenesis-dependent calcified neoplasms using different polymers resulted in very much smaller sizes and narrower polydispersities and that, together with a cathepsin-K-cleavable system, they achieved a more specific drug release and therefore focused the toxicity of the free drugs to the bone tumor (Segal et al., 2009).
In the development of novel nanoscale immunization vector modules (Ag, adjuvant, and carrier) which were assembled into units that were optimized for stimulating immune responses to specific pathogens including the Dengue and West Nile (WN) flaviviruses, Demento et al. (2010) used NTA to help optimize immune responses in mouse.
Corradetti et al. (2012) also used affinity targeted biodegradable nanoparticles to mediate paracrine stimulation as an alternative approach to sustain the growth and pluripotency of mouse embryonic stem cells. They showed sustained release of Leukaemia Inhibitory Factor (LIF) from nanoparticles composed of a solid poly(lactide-co-glycolic acid) polyester or a hydrogel-based liposomal system, which they termed Nanolipogel, replenished once after each cell passage.
Researchers investigating the dendritic cell maturation and T cell activation through the application of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin used scanning electron microscopy, dynamic light scattering, NTA and ultracentrifugation to analyze size, surface charge, and morphology of the nanoparticles (Sokolova et al., 2010).
Dimitrova (2011) has recently described NTA in a discussion on the applications of sub-visible particle analysis in the development of protein therapeutics. Kolluru et al. (2012) also used NTA to develop the optimum formulation of albumin based theragnostic nanoparticles as a potential delivery system for tumor targeting showing that both NTA and DLS confirmed that the optimized nanoparticle formulation had a particle size of 125 nm.
Geng et al. (2012) used NTA to establish that the development and characterizations of maleimide-functionalized biopolymer (Mal-PGA-Asp) as an effective targeted drug delivery carrier, synthesized from an amidation reaction between aspartylated PGA (PGA-Asp) and N-(maleimidohexanoyl)-ethylenediamine (NME), led to significantly enhanced cellular uptake of TP13-Mal-PGA-Asp3-Pt in the human hepatoma cell line SMMC-7721, as shown by fluorescence imaging and flow cytometry. NTA was used to show the biopolymer had an average size 87 ± 28 nm. Satchi-fainaro et al. (2011) have recently patented a novel conjugate of a polymer having a therapeutically active agent and an angiogenesis targeting moiety attached, using NTA data in support of their claim.
Using a range of sophisticated techniques (including matrix assisted laser desorption ionization mass spectrometry (MALDI-TOF), NMR, and high performance liquid chromatography to characterize fullerene derivatives, stabilized FcεRI-mediated mast cells and peripheral blood basophils, both DLS and NTA were used to demonstrate sizes of between 1 and 50 nm in aqueous solution (Dellinger et al., 2013).
Rotan et al. (2013) used NTA to size the calcium phosphate nanoparticles they were using to transport supramolecular drugs across the cell membrane and Petersen et al. (2013) used NTA to size his bioresorbable polymersomes for targeted delivery of cisplatin.
DLS and NTA were used in conjunction to size transferrin(Tf)-containing gold nanoparticles to effect receptor-mediated transcytosis across the blood–brain barrier as a useful way to transport therapeutics into the brain. This transport is aided by tuning the nanoparticle avidity to Tf receptor (TfR), which is correlated with nanoparticle size (45 nm and 80 nm diameter being optimum) and the total amount of Tf decorating the nanoparticle surface (Wiley et al., 2013).
Having previously demonstrated earlier that NTA-analyzed packaging of catalase into a polyion complex micelle (‘nanozyme’) with a synthetic polyelectrolyte block copolymer protected the enzyme against degradation in macrophages and improved therapeutic outcomes, Klyachko et al. (2013) have recently reported the manufacture of nanozymes with superior structure and therapeutic indices and shown that optimized cross-linked nanozyme loaded into macrophages reduced neuroinflammatory responses and increased neuronal survival in mice. Using NTA to measure particle size and concentration, Look et al. (2013) described the nanomaterial-dependent modulation of dendritic cells and its potential influence on therapeutic immunosuppression in lupus, comparing the internalization of two nanoparticulate platforms: a vesicular “nanogel” platform with a lipid exterior, and the widely-used solid biodegradable poly(lactic-co-glycolic acid) (PLGA) system. Although both types of particles could mitigate the production of inflammatory cytokines and the up-regulation of stimulatory surface markers, nanogels yielded greater reductions. These in vitro measurements correlated with in vivo efficacy, where immunosuppressive therapy with nanogels extended the survival of lupus-prone NZB/W F1 mice whereas PLGA particles did not.
The requirement to bridge the gap caused by the diminishing rate of discovery of new biologically active compounds that can be exploited therapeutically to treat disease with fewer new drugs entering the market every year it is now increasingly recognized that the use of nanotechnology in medicine and more specifically drug and gene delivery is set to spread rapidly. There is a requirement to bridge the gap caused by the diminishing rate of discovery of new biologically active compounds that can be exploited therapeutically to treat disease with clinical need. It is increasingly recognized that the use of nanotechnology in medicine, and more specifically drug and gene delivery, is set to spread rapidly
Interest is driven by the knowledge that nanoparticles represent versatile and multifunctional structures for the delivery of drugs allowing better pharmacokinetic properties, controlled and sustained release, and targeting of specific cells, tissues or organs (e.g. in new ways in which to cross the blood-brain barrier) (Malam et al., 2011).
Of specific interest in this area is the use of nanoparticles for transporting and delivering cargoes of genetic material of a wide variety of types. Accordingly, non-viral gene delivery using polymeric nanoparticles has emerged as an attractive approach for gene therapy to treat genetic diseases as well as a technology for regenerative medicine. Unlike viruses, which have significant safety issues, polymeric nanoparticles can be designed to be non-toxic, non-immunogenic, non-mutagenic, easier to synthesize, chemically versatile, capable of carrying larger nucleic acid cargo and biodegradable and/or environmentally responsive.
The delivery of siRNA to cell systems has been the subject of much recent work as a way to enhance human mesenchymal stem cell differentiation via RNA interference (RNAi) which could provide an effective way of controlling cell fate for tissue engineering, but a safe and effective delivery vehicle must first be developed. Tzeng et al. (2012) employed cystamine-terminated poly(beta-amino ester) to this end using NTA to follow size and concentration of different polymer formulations of nanoparticle production. Tzeng and Green (2012) then extended this work to explore subtle changes to the polymer structure and degradation mechanism to such structures for the highly effective short interfering RNA (siRNA) and DNA delivery to human brain cancer.
SiRNA delivery has also been studied through the use of cell-penetrating peptides (CPPs) which are short cationic peptides that have been extensively studied as drug delivery vehicles for proteins, nucleic acids and nanoparticles. They showed a newly developed CPP, PepFect 14 (PF14), forms non-covalent nanocomplexes with siRNA which are able to elicit efficient RNAi response in different cell-lines. NTA was used to demonstrate stability of the nanoparticles on drying and re-suspension (Ezzat et al., 2012).
Similarly, Troiber et al. (2012) compared four different particle sizing methods for siRNA polyplex characterization given no standard technique for size measurements is available. Four different analytical methods were evaluated for their suitability to analyze the characteristics of homogeneous and heterogeneous siRNA polyplexes: DLS, AFM, “nanoparticle trafficking analysis” (NTA) and fluorescence correlation spectroscopy (FCS). While the smallest 40 nm particles were of too low a refractive index to be tracked by NTA, larger particles of 120 nm could be sized by all methods.
More recently, Sunshine et al. (2012), in developing safe and effective delivery system based on poly(beta-amino ester)s which show great potential as gene delivery reagents because they are easily synthesized and they transfect a wide variety of cell types with high efficacy in vitro, have used NTA to determine particle size just prior to subretinal injection. The successful transfection of the RPE in vivo suggested that these nanoparticles could be used to study a number of genetic diseases in the laboratory with the potential to treat debilitating eye diseases.
In the area of the development of nanoparticles as gene delivery vehicles, Ghonaim and his co-workers have reported extensively on the use of NTA in their work on the effect of modifications to the chemistry of lipopolyamines and spermines in various non-viral plasmid DNA and siRNA delivery systems (Ghonaim et al., 2007a, 2007b and 2007c; Ghonaim, 2008; Ghonaim et al, 2009; Soltan et al., 2009; Ghonaim et al., 2010). Similarly, Ofek et al. (2010) have employed NTA for the characterization of dendritic nanocarriers for siRNA delivery while Bhise measured particle size and size distribution by NTA in their study of gene delivery polymer in cell culture (Bhise et al., 2010). Bhise recently further extended this work to develop an assay for quantifying the number of plasmids encapsulated by polymer nanoparticles using NTA to determine the number density of plasmids per 100 nm nanoparticle (Bhise et al., 2010).
Recent work has employed NTA to show that dendrimer structures being used as vehicles for siRNA delivery underwent changes in size and polydispersity at higher dendrimer concentrations which indicated that electrostatic complexation results in an equilibrium between differently sized complex aggregates (Jensen, 2011). This work allowed the optimum dendrimer structure to be identified for subsequent nucleic acid delivery. In another example, the self-aggregation of polyamidoamine dendrimers with hydroxyl surface groups was detected by NTA in Ciolkowski’s study of the influence of PAMAM-OH dendrimers on the activity of human erythrocytes (Ciolkowski et al., 2011).
Sander et al. (2013) and Stremersch (2013) have both reported on the encapsulation of siRNA into extracellular vesicles by electroporation. Sander suggested electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles while Stremersch found this phenomenon is biased by siRNA precipitation.
NTA was used by Shimizu et al. (2013) for the determination of size and number of vesicles used in a study of the expression of miR-145 in Children with Kawasaki Disease. Such microRNAs are small non-coding RNAs that modulate gene expression at the post-transcriptional level and can be transported between cells in extracellular vesicles.
The synthesis and characterization of a novel, bioreducible linear poly(β-amino ester) designed to condense siRNA into nanoparticles and efficiently release it upon entering the cytoplasm was reported by Kozielski et al. (2013), in which NTA was used alongside a zeta potential/DLS method to characterize the nanoparticles. Delivery of siRNA using this polymer achieved near-complete knockdown of a fluorescent marker gene in primary human glioblastoma cells with no cytotoxicity.
Ballarín-González et al. (2013) used NTA to determine the hydrodynamic size of chitosan/siRNA complexes as used in their study by direct Northern and quantitative PCR (qPCR) detection, stability, gastrointestinal deposition, and translocation into peripheral tissue of nonmodified siRNA after oral lavage of chitosan/siRNA nanoparticles in mice. In contrast to naked siRNA, retained structural integrity and deposition in the stomach, proximal and distal small intestine and colon was observed at 1 and 5 hours for siRNA within nanoparticles, indicating an oral delivery platform that could have the potential to treat local and systemic disorders by siRNA.
Gene therapy utilizing lentiviral vectors constitutes a real therapeutic alternative for many inherited monogenic diseases. Therefore, the generation of functional vectors using fast, non-laborious and cost-effective strategies is imperative. Among the available concentration methods for VSV-G pseudotyped lentiviruses to achieve high therapeutic titres, ultracentrifugation represents the most common approach. However, the procedure requires special handling and access to special instrumentation, it is time-consuming, and most importantly, it is cost-ineffective due to the high maintenance expenses and consumables of the ultracentrifuge apparatus. Papanikolaou et al. (2013) have recently described an improved protocol in which vector stocks are prepared by transient transfection using standard cell culture media and are then concentrated by ultrafiltration, resulting in functional vector titres of up to 6x109 transducing units per milliliter (TU/mL) without the involvement of any purification step. They determined the viral functional titre by employing flow cytometry and evaluated the actual viral particle size and concentration in real time using NTA.
Similarly, adenoviral vectors hold immense potential for a wide variety of gene therapy based applications; however, their efficacy and toxicity is dictated by “off target” interactions that preclude cell-specific targeting to sites of disease. In order to overcome these limitations, Parker et al. (2013) have developed capsid modification strategies for detargeting Adenoviral vectors, using NTA to determine viral titre and stability.
NTA has also been used in the development of non-viral gene delivery systems based on a lipophilic plasmid DNA condensate (Do et al., 2011), poly(β-amino ester)s (PBAEs) (Tzeng et al., 2011) and in the screening of such structures in vitro (van Gael et al., 2011). More recently, in their evaluation of polymeric gene delivery nanoparticles by NTA and high-throughput flow cytometry, Shmueli et al. (2013) described a new protocol to characterize PBEA nanoparticles utilizing NTA. Such PBAEs are hydrolytically degradable and have been shown to be effective at gene delivery to hard-to-transfect cell types such as human retinal endothelial cells, mouse mammary epithelial cells, human brain cancer cells and macrovascular (human umbilical vein) endothelial cells. This NTA-based protocol was considered to be easily adapted to evaluate any polymeric nanoparticle and any cell type of interest in a 96-well or multi-well plate format for transfection assay for rapid screening of the transfection efficacy. Bhise et al. (2013) also evaluated, using DLS and NTA, the potential of poly (beta-amino ester) nanoparticles for reprogramming human fibroblasts to become induced pluripotent stem cells. Evaluating the use of a biodegradable PBAE nanoparticle-based nonviral protocol and comparing it with an electroporation-based approach to deliver episomal plasmids encoding reprogramming factors for generation of human induced pluripotent stem cells from human fibroblasts, they screened a polymer library to identify the polymers most promising for gene delivery to human fibroblasts. They concluded, however, that certain nonviral reprogramming methods may not necessarily be safer than viral approaches and that maximizing exogenous gene expression of reprogramming factors is not sufficient to ensure successful reprogramming. The same group also evaluated the uptake mechanism of PBAE polyplexes and the dependence of cellular uptake on the end group and molecular weight of the polymer by synthesizing three different analogues of PBAEs with the same base polymer poly(1,4-butanediol diacrylate-co-4-amino-1-butanol) but with small changes in the end group or molecular weight. They showed that differential polymer structure tunes the mechanism of cellular uptake and transfection routes of poly(β-amino ester) polyplexes in human breast cancer cells (Kim et al., 2013).
Troiber (2013) has described sequence-defined polycationic oligomers for nucleic acid delivery, in which NTA was considered ideal for the measurement of medium sized particles such as 332 polyplexes which displayed a mean diameter of 139±47 nm and in which the resolution of NTA was therefore higher than for DLS.
Another method of separating nucleic acid polymer conjugates has recently been patented and in which NTA data was reported (Case, 2013).
Witwer et al. (2013) have suggested that real-time quantitative PCR (RT-qPCR) and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs in contrast to previous evidence that exogenous dietary miRNAs enter the bloodstream and tissues of ingesting animals and which has been accompanied by an indication that at least one plant miRNA, miR168, participates in “cross-kingdom” regulation of a mammalian transcript. Employing RT-qPCR to measure plant and endogenous miRNAs from pigtailed macaques that received a miRNA-rich plant based substance, their results did not support general and consistent uptake of dietary plant miRNAs. NTA was used to show a shift in particle size and population following food intake.
In a sophisticated study using an X-ray free-electron laser, Demirci et al. (2013) carried out femtosecond X-ray diffraction of 30S ribosomal subunit microcrystals in liquid suspension at ambient temperatures, confirming crystal concentration was approximated as 1010 -1011 /milliliter by NTA. Such high-resolution ribosome structures determined by X-ray crystallography have provided important insights into the mechanism of translation.
Mostaghaci et al. (2013) have developed a one-step synthesis of nano-sized and stable amino-functionalized calcium phosphate (low toxicity with simple and low cost synthesis) particles for DNA transfection, having used NTA to confirm that their refined wet-precipitation method yielded NPs with a narrow size distribution (~140 nm), a significant improvement on the previous results in which the transfection results varied because the precipitation lacked reproducibility and resulted in poly-dispersed, agglomerated particles.
Chitosan-based nanoparticles were also studied using NTA for gene-and siRNA-delivery (Malmo, 2012) and as permeating vectors for the blood–brain barrier when functionalized with alkylglyceryl (Lien et al., 2012).