Solid-state characterization


Comprehensive solid-state characterization equips drug developers and manufacturers with critical insights to bring pharmaceutical products to market. Explore our solutions for analyzing drug compounds in their solid state.
Particle size and shape are critical material attributes for all solid dosage forms and liquid suspensions, determining crucial parameters such as bioavailability and processability. The measurement of particle size, shape, and size distribution is therefore essential throughout the drug development and manufacturing process.

Particle sizing is critical at multiple stages of pharmaceutical development and production, and for years, the Mastersizer range has been the trusted choice for industry professionals.
The Mastersizer 3000+ is designed to address the key challenges of API particle sizing while meeting the rigorous demands of regulatory and quality frameworks. Building on decades of expertise in laser diffraction, it introduces advanced software features that elevate particle-sizing capabilities:
With the Mastersizer 3000+, you gain the tools and confidence to tackle the most complex particle-sizing challenges.
Control particle size and shape with precision during formulation and scale-up.
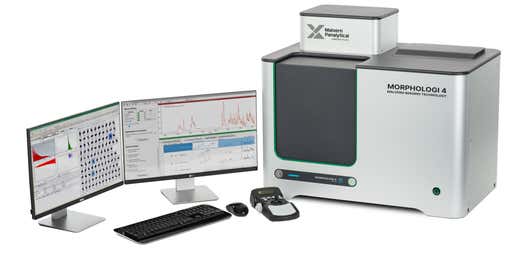
The Morphologi range delivers fully automated static image analysis. The Morphologi 4-ID combines automated static image analysis with Raman spectroscopy to deliver detailed chemical and morphological characterization of individual particles, even in complex samples. It also validates measurements from Mastersizer 3000+ for seamless integration into your workflow.
Optimize manufacturing with real-time particle size analysis using the Insitec range. Designed for high-throughput environments, Insitec provides continuous laser diffraction monitoring for solids (Insitec Dry) and liquid suspensions (Insitec Wet).
Capable of analyzing up to 20kg of solid-state compounds per hour, Insitec enables quick identification of deviations, turning potential batch losses into routine corrections, saving time and costs.
Understanding the crystal structure of drug substances is essential for ensuring stability, solubility, bioavailability, and polymorphic consistency. X-ray diffraction provides the insights needed to optimize the solid form of active pharmaceutical ingredients (APIs), achieve stable drug formulations, streamline production, and ensure product quality across the drug product lifecycle.
If you doubt the homogeneity of your materials or have evidence of sample inconsistency, then you should explore the benefits of Morphologically-Directed Raman Spectroscopy. This technology combines analytical imaging with Raman Spectroscopy in the Morphologi 4-ID.


Aeris simplifies crystal structure analysis for monitoring polymorphism, solid form stability and crystallinity/amorphicity of drug substances. XRD data collected on Aeris can also be used for Limit of Detection (LOD) and Limit of Quantification (LOQ) assessments that support reliable analysis of polymorphic contaminations and pave the way for robust QC batch release testing. Aeris can support you throughout drug substance development through validation of solid form on solid
What’s more, OmniTrust software ensures 21 CFR Part 11 compliance, while Aeris’ modular design and vast analytical capabilities of HighScore software future-proof your investment.
For advanced crystallographic and polymorphic studies, Empyrean is the ultimate solution:
Empyrean meets the needs of both research and manufacturing, providing tailored solutions for X-ray diffraction studies.
When you need to screen samples fast, X-ray fluorescence analyzers deliver highly accurate analysis of high-concentration samples, making them particularly useful for process chemists working on purification, catalyst scavenging, and synthesis optimization.
Energy-dispersive (ED) XRF is a fast, cost-effective, and user-friendly alternative to traditional ICP methods for screening elemental impurities in pharmaceutical samples, delivering reliable results that comply with USP <232,233> and Q3D.
Revontium is the world’s first compact ED XRF analyzer, delivering floor-standing power in a 0.4m² footprint.
Revontium enhances your elemental analysis capabilities, making it more efficient and accessible.
The Epsilon Energy Dispersive XRF (ED XRF) analyzers make in-house elemental analysis simple and cost-effective:
Our decades of experience supporting customers and working with partners in the pharmaceutical give us a keen insight into the challenges you face throughout drug development and manufacturing processes. As a result, our analytical solutions are used by major pharmaceutical companies to support their processes from drug discovery to quality control, as well as in peer-reviewed research.
Our extensive portfolio of solutions covers every step of the drug development and manufacturing workflow, supported by a wide range of support services, from method development support to proactive instrument monitoring via Smart Manager.
Contact us to find out more about how we can support your solid-state characterization.
